Chapter 12. Chemotherapy
SECTION A Basic Chemotherapy Principles
Carlos O. Rodriguez Jr.
KEY POINTS
• By the time tumors are clinically detectable, they contain a significant number of mutations, rendering those cells inherently resistant to many chemotherapeutic agents.
• Cytotoxic drugs should be given at their maximally tolerated dose as often as possible to maximize their efficacy.
The goal of chemotherapy is to reduce the number of malignant cells in a patient to zero. Two theories have been developed predicting cell kill with cytotoxic therapy: the fractional cell kill hypothesis, and the Norton-Simon hypothesis. The Norton-Simon hypothesis relates the effect of cytotoxic therapy to the growth dynamics of the tumor. In other words, the response of a tumor to chemotherapy is proportional to the tumor growth rate. 1 According to the fractional cell kill hypothesis, a given drug will kill a constant fraction of the cell population, regardless of the absolute number of cells in that population. 2-4 Putting this into clinical context, if a patient with an average-sized tumor of 10 11 cells is treated with a drug that will reduce the number of tumor cells by 99%, 1% or 0.01 of the tumor cells will survive. Theoretically after the first dose of chemotherapy, the remaining number of tumor cells will be 10 11 × 0.01, or 10 9 cells. If five more doses of chemotherapy are given (10 9 × 0.01 × 0.01 × 0.01 × 0.01 × 0.01), the remaining neoplastic population will be 10 7 , 10 5 , 10 3 , 10 1 (one cell), and 10 -1 (one tenth of one cell, or zero cells), respectively. 2-4 Experience amply demonstrates that this theory rarely translates into clinical reality, but why? There are myriad factors. These can be divided into tumor-related and pharmacological considerations.
TUMOR-RELATED CELL KILL CONSIDERATIONS
The kinetics of the cell cycle plays a role in determining the sensitivity of a population of cells to chemotherapeutic agents. Neoplastic and normal cells that are actively proliferating are considered to be “in the cell cycle.” The cell cycle is divided into four phases: G 1, S, G 2, and M phase. 5,6 See Figure 12-1 for an illustration of the cell cycle. Terminally differentiated cells (such as neurons or small lymphocytes) are considered to be in a resting phase outside of the cell cycle termed G 0 . Poorly oxygenated tumor cells are often in this phase as well. The growth of tumors has been characterized by Gompertzian growth kinetics 4 ( Figure 12-2 ). According to this model, the fraction of proliferating, and therefore drug-sensitive, cells is not constant. A 1-cm, clinically detectable tumor contains 10 9 tumor cells. 7 At low population numbers, <10 9 cells, the cells are most likely to be in the cell cycle and therefore most sensitive to chemotherapy. By the time a tumor is clinically detectable, the tumor cell population is actually entering a phase of decelerating growth. Because chemotherapeutics are most effective against rapidly dividing cells, having cells in the G 0 phase of the cell cycle results in poor cell killing by the administered chemotherapeutics. 2-4
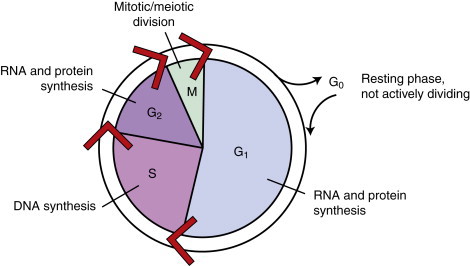 |
| FIGURE 12-1 The cell cycle is a continuous process. The phases of the cell cycle and the corresponding activities of each phase are shown. |
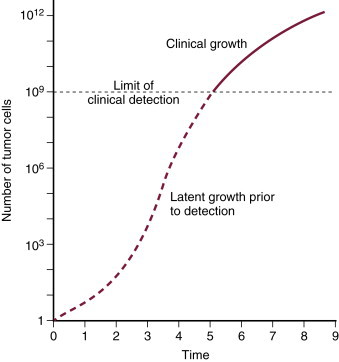 |
| FIGURE 12-2 Gompertzian growth kinetics. According to this model of tumor growth, the fraction of proliferating tumor cells is not constant. A clinically detectable tumor is approximately 1 cm in diameter and contains 10 9 tumor cells. Tumors experience exponential growth prior to this, and once they contain more than 10 9 cells, their growth decelerates as shown by the plateau on the growth curve. Tumor cells are most sensitive to chemotherapy and radiation therapy since they are rapidly dividing prior to becoming clinically detectable. (From Slingerland JM, Tannock IF: Cell proliferation and cell death. In Tannock IF, Hill RP [eds]: The basic science of oncology , ed 3, New York, 1998, McGraw-Hill.) |
PHARMACOLOGICAL CELL KILL CONSIDERATIONS
The response of the tumor to chemotherapy also depends upon pharmacokinetic factors such as drug absorption, metabolism, and elimination. These parameters are extremely important in guiding the dose, schedule, and route of administration of the drugs. Few drugs used in veterinary oncology have undergone rigorous clinical pharmacological investigations. One parameter that has been determined for many of the useful drugs is the maximally tolerated dose (MTD). The MTD is the maximum recommended dose of an agent that can be administered safely based on toxicity. The MTD is then administered as often as allowable, which is determined by the recovery of the normal tissues (e.g., bone marrow and gastrointestinal lining). Maximizing the exposure of the tumor to the MTD is important in achieving desired clinical results.
The expected response of a tumor to chemotherapy also depends on the way in which the agent is used. Chemotherapeutic agents may be used in a neoadjuvant or adjuvant setting and can be divided into induction, consolidation/maintenance, or salvage/rescue protocols. Neoadjuvant chemotherapy refers to the use of chemotherapy before primary treatment for the disease. An example is the use of chemotherapy in an effort to cytoreduce a bulky tumor in advance of the definitive local treatment with surgery or radiation therapy. Complete resolution of a bulky tumor with chemotherapy is unlikely because a large tumor is likely to contain only a small fraction of cycling cells that are sensitive to chemotherapy and many cells that have acquired chemotherapy resistance through mutation. Adjuvant chemotherapy is applied after definitive local therapy (surgery, radiation therapy) but where the risk of local recurrence (incompletely excised malignancies) or the likelihood of metastasis (e.g., apocrine gland adenocarcinoma of the anal sac or osteosarcoma) is high. Induction therapy most often applies to the use of chemotherapy to treat lymphoid malignancies. These protocols are intensive because they involve short dosing intervals and aggressive drug combinations. The goal of induction therapy is to achieve a complete remission, or complete resolution of clinically measurable disease. Consolidation therapy is less intensive than induction therapy and may or may not use the same drugs as the induction phase. The goal of consolidation therapy is to continue the cytoreduction of the tumor burden when induction resulted in only a partial response. Maintenance therapy is a continuation of chemotherapy when a complete remission has been achieved in an effort to maintain remission and, thus, prevent relapse. Salvage/rescue protocols are used in patients where standard protocols no longer maintain complete (or partial) remission. In general, the drugs in these protocols are not considered front-line treatments and occasionally have more severe toxicity profiles. Recalling the Goldie-Coleman hypothesis, this population of tumor cells has been subjected to multiple rounds of chemotherapy and, consequently, are likely to have acquired mutations that result in their continued survival.
Most chemotherapy protocols combine multiple drugs at minimal intervals. The Goldie-Coleman hypothesis again provides the rationale for combination chemotherapy: at the time of clinical detection, the presence of drug resistance is almost assuredly guaranteed. When choosing agents for combination chemotherapy protocols, three principles should be applied: (1)each drug should have known cytotoxic activity against the intended tumor, (2) overlapping toxicities should be avoided between drugs, and (3) each drug should be used as near its MTD as possible. 9 In addition, each drug should possess different mechanisms of action from one another. Table 12-1 lists chemotherapy drugs and the phase of the cell cycle within which they function, which is also an important factor when combining chemotherapy drugs. Dose intensity , the amount of drug delivered over time (mg/m 2 /week), should be maximized by administering the MTD as often as possible for each drug so there is a greater chance of killing both sensitive and moderately resistant cells in the heterogeneous tumor population.
| ∗ Some classes of chemotherapy drugs exert their mechanisms of action during specific phases of the cell cycle. The alkylating agents, anthracyclines, and platinum agents are non-cell cycle phase–specific exerting their effects throughout the cell cycle. Cell cycle phase specificity is considered when developing multi-agent chemotherapy protocols. | |
| Cell Cycle Phase | Chemotherapy Drug Class |
|---|---|
| G 1 | l -Asparaginase |
| S | Antimetabolites |
| G 2 | |
| M | Vinca alkaloids |
It is the hope of the author that the above concepts, theories, and discussions will translate into an increased appreciation of what is happening (or not happening) when a chemotherapy drug is administered. These concepts are crucial to the understanding of the theory of chemotherapy and its application and are as important as the properties of the individual chemotherapy drugs.
Selected References ∗
V.T. DeVita, P.S. Schein, The use of drugs in combination for the treatment of cancer: rationale and results , N Engl J Med 288 ( 1973 ) 998 ;
This publication describes the rationale to choose cytotoxic agents for combination chemotherapy protocols. .
J.H. Goldie, A.J. Coldman, A mathematical model for relating the drug sensitivity of tumors to their spontaneous mutation rate , Cancer Treat Rep 63 ( 1979 ) 172 ;
This publication provides the rationale behind the hypothesis that each tumor contains mutations making them inherently resistant to chemotherapy. .
H.E. Skipper, F.M. Schabel Jr., L.B. Mellet, et al. , Implications of biochemical, cytokinetic, pharmacologic and toxicologic relationships in the design of optimal therapeutic schedules , Cancer Chemother Rep 54 ( 1950 ) 431 ;
This publication was the first to describe the fractional cell kill hypothesis of tumors with the application of chemotherapy.
SECTION B Chemotherapy Drug Interactions
Wendi Velando Rankin and Carolyn J. Henry
KEY POINTS
• Chemotherapy drugs can interact with other drugs in vitro (such as during mixture or administration) and in vivo .
• Drug interactions can enhance or decrease activity of the chemotherapy drug.
• Drug interactions can result in beneficial effects, severe toxicities, or no detectable clinical change.
• Laboratory tests can be altered with some chemotherapy drugs.
Treatment of a cancer patient with chemotherapy drugs can be complex, since patients may be receiving supportive therapy or other drugs for treatment of concurrent disease conditions, including systemic and topical therapies (ophthalmic and otic preparations). In addition, chemotherapy often requires multiple drug therapy (polypharmacy) for more efficacious treatment of neoplastic diseases; therefore, knowledge of drug interactions is important for safe drug administration.
Chemotherapeutics can interact with other drugs both in vitro (outside the body) and in vivo (inside the body). The results of these drug interactions may vary from beneficial to severely adverse reactions, or there may be no detectable clinical change. To prevent detrimental effects of drug interactions, it is important to be familiar with potential adverse reactions, since some reactions are unpredictable.
MECHANISMS OF DRUG INTERACTIONS
In vitro interactions may occur when incompatible drugs are mixed together before administration, such as in the same intravenous fluids. This can result in alteration of the chemical nature of one or more of the drugs, leading to decreased activity of the drug. Some of the incompatibilities manifest as visible changes, including a precipitate or haze from the drug reactions, an increase or decrease in the natural turbidity of the drug, gas evolution, or color change of the solution ( Figure 12-3 ). For example, consecutive injections of doxorubicin and heparin into a fluid line without flushes between them results in immediate precipitation. 1-9 In addition, mixtures of paclitaxel and amphotericin B in vitro result in a decrease in the natural turbidity of the solution, 8,9 and fluorouracil in a solution with doxorubicin results in an immediate color change from red to purple. 3-5,6,9 A mixture of carmustine and allopurinol in a fluid line results in gas formation. 9 On the other hand, the reactions can be more subtle and result in a change in drug activity without visible signs; some drug interactions will change the spectrophotometry of a drug solution as a result of altered drug activity, and this will not be an overtly visible change. Chemical decomposition of the drug can also occur in a mixture without detectable visible changes, and sometimes can only be detected by purification of the agent. Fluorouracil combined with methotrexate in vitro results in a change in the spectrophotometry of both agents, 3-7 and this is not visibly apparent. In addition, cisplatin in solutions with low chloride content (<0.2% sodium chloride) will result in a displacement of the chloride ions by water, resulting in inactivation of the agent 3,5,7-9 as a result of the addition of a positive charge ( Figure 12-4 ). The positive charge prevents the movement of cisplatin into cells. Many drug interactions can go unnoticed if there is no visible color change or precipitate; therefore, avoid mixing drugs for which there are no compatibility data.
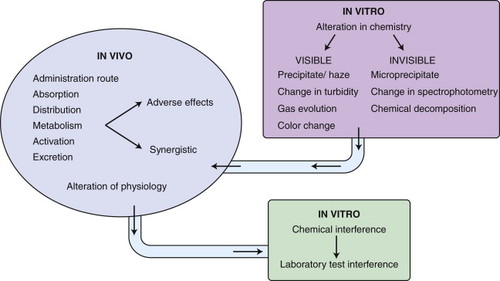 |
| FIGURE 12-3 Mechanisms of drug interactions. |
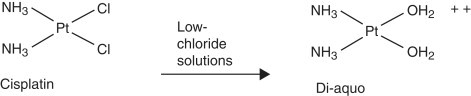 |
| FIGURE 12-4 Cisplatin in low-chloride solutions (e.g., dextrose 5% in water, <0.2% sodium chloride) will allow chloride groups to leave. They are replaced by water, and the resulting compound has a charge and cannot enter cells for its anti-tumor effects. Pt , Platinum; Cl , chloride; NH 3 , ammonia. |
In vivo drug interactions may enhance or decrease drug activity and toxicity depending on various factors. These drug interactions depend on factors that affect the administration route, absorption, distribution of the drug, metabolism and activation, or excretion of drugs. For example, intravenous melphalan may reduce the threshold for carmustine-induced pulmonary toxicity 8 ; however, this is not reported with oral melphalan and carmustine. In addition, food given with oral methotrexate will result in decreased absorption of methotrexate and therefore decreased bioavailability and efficacy of the chemotherapeutic. 10 Therefore, methotrexate should not be given with food. Cyclosporine decreases doxorubicin clearance and allows better distribution inside cells because of inhibition of the P-glycoprotein drug efflux pump. 5 Although this can allow better anti-tumor effects, it can also potentiate hematologic toxicity and neurotoxicity. 8,11 One drug can affect the metabolism or excretion of another, as with cyclophosphamide and barbiturates. Patients receiving concurrent barbiturates and cyclophosphamide can have increased metabolism of cyclophosphamide to its active metabolites because of barbiturate induction of hepatic microsomal enzymes 2,4,5,7,8 ; this increases cyclophosphamide toxicity. Cimetidine can decrease the hepatic degradation of 1-(2-chloroethyl)-3-cyclohecyl-1-nitrosurea (CCNU) resulting in increased myelosuppression of the chemotherapeutic. 5,11 Cimetidine inhibits hepatic microsomal enzymes, decreases hepatic blood flow, and results in increased bioavailability of drugs (such as CCNU) with high hepatic excretion. 8 Cisplatin given concurrently with methotrexate results in increased nephrotoxicity because of cisplatin decreasing the renal clearance of methotrexate. 5,7 Although many interactions can have adverse effects as described previously, the interactions can also be used to our advantage for anti-tumor effects, including fluorouracil and cisplatin, 7,8 chlorambucil and prednisone for leukemia, 4,7,8 and cisplatin and bleomycin given together. 8 However, note that some drug combinations have both synergistic as well as adverse effects. For example, whereas cisplatin and bleomycin have synergistic anti-tumor effects, 8 cisplatin decreases the renal clearance of bleomycin 5 and can increase bleomycin toxicity. 7,12
Lastly, some interactions are in vitro as well as in vivo . Drugs may interact with laboratory tests due to the drug effects on the analysis method (in vitro) or altering the body’s physiologic response (in vivo), resulting in false results ( Table 12-2 ). For example, patients receiving mercaptopurine can have falsely elevated blood glucose and uric acid measurements because of the drug’s interference in vitro with the laboratory analyzer. 8 In addition, patients who recently received l-asparaginase may have falsely low-serum thyroxine measurements because of decreased synthesis of thyroxine in vivo . 8
| Drug | Laboratory Test Abnormalities | Comments |
|---|---|---|
| Actinomycin D | Interference with antibacterial drug levels 8,11 | Actinomycin D may interfere with bioassay |
| Carboplatin | Abnormal liver function tests 8 | With high doses of carboplatin (>four times dosage) |
| Hydroxyurea | Increased serum uric acid, blood urea nitrogen, creatinine 8 | |
| l -Asparaginase | Rapid and marked reduction in serum thyroxin-binding globulin, reduction in total serum thyroxine 8 ; leads to increased thyroxine-binding globulin index 11 | Occurs within 2 days after first dose; serum concentrations of thyroxine-binding globulin returned to pre-treatment values within 4 weeks 8 ; may result from asparaginase-induced inhibition of serum thyroxine synthesis in liver 11 |
| Mercaptopurine | Falsely elevated serum glucose and uric acid levels 11 | Mercaptopurine interferes with sequential multiple analyzer 12/60 determinations |
| Mitotane | Decreased protein-bound iodine 8,11 | Mitotane competitively binds thyroxine-binding globulin and decreases serum protein-bound iodine 11 |
| Decreased urinary 17-hydroxycorticosteroids 8,11 | Increased extra-adrenal metabolism of cortisol to 6-β-hydroxycortisol; may not reflect decrease in cortisol secretion rate or plasma cortisol concentration | |
| Phenytoin | Interference with dexamethasone-suppression tests 11 | |
| Prednisone | Decreased 131 I uptake and protein-bound iodine concentrations 11 |
SPECIFIC DRUG INTERACTIONS
Tables included on the website www.smallanimaloncology.com list drug interactions with various chemotherapy agents used in veterinary clinical oncology. The information focuses on agents applicable to veterinary medicine but is not an exhaustive list of all possible interactions. Chemotherapy drugs with no known incompatibilities are not discussed. Although herbal medications and nutritional supplements are increasing in popularity as alternatives or adjuncts to cancer therapy, because they are not currently used as standard therapy for our veterinary patients, interactions with these supplements are not included. However, veterinarians should understand that any drug or supplement can have potential drug interactions and may affect treatment of a patient.
Table 12-A1 on the website summarizes interactions with chemotherapy agents; drugs are listed based on drug class. Table 12-A2 on the website summarizes reported physical in vitro incompatibilities of chemotherapy agents with other drugs; drugs are listed based on drug class. Table 12-2 in this chapter is a summary of known laboratory test interferences of which clinicians should be aware; drugs are in alphabetical order. Although the interactions listed in these tables summarize the reported interactions, much of the data is obtained from human pharmacology books; therefore, the clinical relevance to veterinary patients is unknown. In addition, the clinical effects of some in vitro drug incompatibilities listed have not been evaluated in veterinary patients.
Selected References ∗
B.A. Chabner, D.L. Longo, Cancer chemotherapy and biotherapy: principles and practice . ed 3 ( 2001 ) Lippincott, Williams & Wilkins , Philadelphia ;
A textbook on principles of chemotherapy and chemotherapy drug information. .
R.T. Dorr, D.D. Von Hoff, Cancer chemotherapy handbook . ed 2 ( 1994 ) Appleton & Lange , Norwalk ;
A textbook on administration of chemotherapy in human patients and individual drug information, including drug interactions for each drug. .
D.S. Fisher, M.T. Knobf, H.J. Durivage, Drug interactions with antineoplastic agents , In: (Editors: D.S. Fisher, M.T. Knobf, H.J. Durivage) The cancer chemotherapy handbook ed 4 ( 1993 ) Mosby , St Louis ;
A manual on chemotherapy principles, administration, and drug interactions. .
C.J. Henry, W.J. Brewer, Drug interactions with antineoplastic agents , In: (Editor: J. Bonagura) Kirk’s current veterinary therapy XII ( 1995 ) WB Saunders , Philadelphia ;
This chapter lists chemotherapy agents and drugs with which they interact in vivo as well as in vitro incompatibilities focusing on drugs more commonly used in veterinary medicine. .
In: (Editor: G.K. McEvoy) Antineoplastic agents American Hospital Formulary Services (AHFS) Drug Information , ( 2005 ) American Society of Health-System Pharmacists , Bethesda ;
A pharmaceutical manual providing details of individual drug information, including pharmacology, use, toxicities, drug interactions, incompatibilities, stability, and chemistry of agents.
SECTION C Safe Handling of Chemotherapy Drugs
Natalie S. Royer
KEY POINTS
• Appropriate personal protective equipment should be worn by all persons handling chemotherapy and patients receiving chemotherapy.
• Patient excrement should be handled as contaminated waste for 48 hours after chemotherapy administration.
• Dogs should be walked in low-traffic sunlit areas for 48 hours after chemotherapy.
• Litter box liners should be used and litter changed daily for 48 hours after chemotherapy.
Safety is of utmost importance when handling cytotoxic agents. The cytotoxic agents used in veterinary medicine are primarily human-approved drugs used off label in the veterinary setting. Therefore, toxicity is possible if these drugs are absorbed or ingested by veterinary personnel. Because of this, the clinical use of cytotoxic agents requires an understanding of associated risks by all personnel involved with chemotherapy preparation, administration, and patient handling. The Occupational Safety and Health Administration (OSHA) has developed guidelines for handling of cytotoxic agents, and these guidelines—available at www.osha.gov/dts/osta/otm/otm_vi/otm_vi_2.html —should be available for all personnel to review prior to their involvement in chemotherapy administration.
PERSONAL PROTECTIVE WEAR
All personnel involved with chemotherapy administration should wear protective clothing. Chemotherapy gloves or a double layer of powder-free latex gloves, eye protection, and a closed-front, elastic-cuffed, non-permeable, lint-free gown are recommendations of OSHA for personal protective equipment for individuals handling hazardous drugs, including cytotoxic agents ( Box 12-1 ). These recommendations should be followed not only by those administering chemotherapy, but also by those restraining animals for treatment. Chemotherapy gloves that are powder free and are thicker than typical examination gloves are commercially available. The thickness of the gloves is most important, and latex has been found to be the least permeable glove material. Powder-free gloves are recommended, since powder may absorb contaminants and thereby potentially increase exposure to cytotoxic drugs. Double-gloving is recommended if specific chemotherapy gloves are not used. Hands should always be washed before gloves are put on and immediately after they are removed. Gowns should be disposable with a closed front and made from low-permeability fabric. The sleeves should be long with elastic or knit cuffs. Gloves should be placed over the cuff unless double gloves are used; then the inner layer of gloves should be under the cuff and the outer over the cuff. Personal protective equipment should also be worn when cleaning up accidental spills or patient waste during the first 48 hours after administration of chemotherapy medications. If clients are to administer chemotherapy such as oral cyclophosphamide or chlorambucil at home, powder-free latex gloves should be dispensed for the client to wear to administer the tablets.
BOX 12-1
RECOMMENDED PERSONAL PROTECTIVE EQUIPMENT FOR HAZARDOUS DRUG HANDLING
Powder-free latex gloves
Closed-front disposable gown of lint-free, low-permeability fabric with long sleeves and cuffs
Chemical-barrier face and eye protection
CHEMOTHERAPY ADMINISTRATION SAFETY
Administration of chemotherapy should always be done in a low-traffic, controlled airflow area such as an examination room or radiology room with a sign on the door warning personnel that chemotherapy is being administered. No food storage, eating, drinking, gum-chewing, smoking, or make-up application should be performed in the area. Heavy make-up wearers and smokers need to be especially cautious, since chemotherapy will adhere to make-up and nicotine on hands and faces.
Equipment necessary for chemotherapy administration is listed in Box 12-2 . Before drug infusion, a plastic-backed absorbent pad should be placed under the animal and injection site to absorb any drug that may be lost accidentally during the administration procedure. When IV administration is performed, the use of an alcohol-soaked gauze around the injection connection will help to trap any chemotherapy that may leak from the connection. Removal of needle caps with your teeth or recapping needles should be avoided at all times. Luer-Lok syringes are recommended for cytotoxic drugs as they help to reduce leakage of drugs and accidental disconnections during drug administration, thus, decreasing the potential for personnel exposure. Using Luer-Lok fittings will decrease the need for needles during chemotherapy administration and significantly decrease the potential for personnel exposure. See Section E for discussion of chemotherapy administration techniques.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


