CHAPTER 9 CHELONIANS
COMMON SPECIES KEPT IN CAPTIVITY
There are over 285 different species of chelonians in the world.1 Chelonians represent one of the most unique and recognizable groups of animals in the world. Characterized by their bony shell, no other tetrapod has both its pectoral and pelvic girdles encased in bone.1 The chelonians are the longest lived of the reptiles, with some animals living over 100 years. Over the years, chelonians have been classified using a number of different taxonomic classifications, including morphologic and molecular features. However, for the purpose of this text, we will classify them based on habitat selection. For the captive chelonian, this is a preferred method because it is useful for defining the captive needs of the chelonian. The two primary classifications based on this system are the turtle and the tortoise. Turtles include species that are aquatic. These animals generally leave the water only to move to another body of water or to lay their eggs. Examples of this group can be found in Box 9-1 and Figures 9-1 and 9-2. For this group it is important to determine what type of aquatic system the animals originate from. There are three different types of aquatic systems: freshwater, brackish, and marine systems. The provision of the most appropriate system is critical to the long-term care of these animals in captivity. The tortoises include animals that are completely terrestrial. These animals are generally considered poor swimmers, and if provided a deep body of water in captivity, could drown. Common examples of these animals can be found in Box 9-1 and Figures 9-3 and 9-4.
BOX 9-1 Common Species of Chelonians Kept in Captivity (pet and institutional)
Turtles
| Alligator snapping turtles | Macroclemys temminickii |
| Snapping turtles | Chelydra serpentina |
| Red-eared slider | Trachemys scripta elegans |
| Diamond-backed terrapins | Malaclemys terrapin |
| Soft-shelled turtles | Apalone spp. |
| Mata mata | Chelus fimbriatus |
BIOLOGY
Integumentary System
The epidermis is composed of an outer keratinized layer (stratum corneum) and an inner actively dividing layer (stratum germinivatum). The dermis represents the vascular region of the integument and supplies nutrition to the epidermis. The dermis is also the location of the osteoderms. There are limited glandular structures within the integument of chelonians. Ecdysis tends to be random and uncoordinated and results in fragmentary shedding, which continues throughout the life of the chelonian.2
The majority of the chelonian shell is comprised of membranous bone (osteoderms). The membranous bone is covered by horny scutes, which form the outer, visible part of the shell. The scutes are formed from the epidermis and are equivalent to the scales of reptilian skin (Figure 9-5). Growth of the scutes occurs by the addition of new keratinized layers to the base of each scute.3 The shell is a vascular structure. In the past, recommendations were made to subtract the weight of the shell when determining drug doses; however, this is not recommended, as the shell is a living tissue and should be included when calculating a therapeutic plan.
Cardiovascular System
The chelonian heart lies in a pericardial cavity and is located slightly caudal to the pectoral girdle and cranial to the lungs.3 Externally, the heart is generally located where the humeral and thoracic plastron scutes meet. The heart is three chambered, with two separate atria and an incomplete ventricular septum. The right atrium receives deoxygenated blood from the left and right precaval veins, postcaval vein, left hepatic vein, and other, smaller vessels. The left atrium receives blood from theleft and right pulmonary veins. Numerous muscular folds in the walls of the ventricle shunt oxygenated and deoxygenated blood into the appropriate arterial pathways.2 The left aorta gives rise to the celiac, left gastric, and cranial mesenteric arteries before converging with the right aorta.3
Renal Portal System
Venous return from the pelvic limbs drains into the kidneys to form the renal portal system in reptiles.3 The primary function of the renal portal vein is to maintain adequate blood flow to the renal tubules when the glomerular blood flow is low. This large vessel arises near the junction of the epigastric and external iliac veins and enters the kidney centrally.2 The chelonian renal portal vein contains valves that effectively shunt blood drained from the caudal half of the body directly into either the kidney or the liver and central venous reserve.4–6
Although avoidance of the hindlimbs for intramuscular and intravenous injections of drugs has traditionally been advocated, research has demonstrated that this may be an unsubstantiated theory. Results of research conducted by Holtz et al. demonstrated that it is unlikely that the injection site has any influence over the activity of a drug and that the caudal half of the reptile body is suitable for drug administration.5 Both Beck et al. and Holtz et al. found no significant difference in drug metabolism when gentamicin (excreted by glomerular filtration) was administered into the hindlimb, as opposed to the forelimb.5,7 Studies conducted by Malley revealed that adrenaline released during caudal intramuscular injections may reduce perfusion of the renal portal system, thereby increasing hepatic circulation.8 It is important to consider that these studies were conducted on a limited number of species. Additional research is required to further elucidate if there is an effect based on species or an animal’s physiologic status.
The Dive Reflex
It has been suggested that the pathway of blood flow from the heart into the pulmonary vessels is under some degree of physiologic control. The dive reflex in anesthetized turtles (Trachemys scripta) has been studied intensely, and it was consequently discovered that hypoxia resulted in an increase in the resistance of pulmonary blood vessels. Because this effect persisted in the face of atropine administration and cervical vagotomy, locally mediated resistance was suggested. Considering the fact that chelonians have only one ventricle, thereby limiting the separation of oxygenated and deoxygenated blood, alterations in vascular resistance may have a significant effect on the perfusion of organs, including the lungs. Consequently, the rates of elimination of inhalation agents by the lungs may be reduced in those situations where the dive reflex is observed.9
Respiratory System
UPPER RESPIRATORY TRACT
Chelonians are obligate nasal breathers.3 The nares open into a keratinized vestibule, which is divided cranially by a cartilaginous septum into right and left nasal chambers. The nasal chambers do not contain either turbinates or sinuses and extend caudally until converging into a single passageway dorsal to the hard palate. The passageway terminates at the choana. A soft palate is not present in chelonians.10,11
LOWER RESPIRATORY TRACT
The chelonian glottis is easily identified at the back of the tongue. The tracheal rings are complete3 and the trachea, bronchi, and lungs are all covered by a ciliated glandular epithelium that is ineffective at eliminating particulate foreign material and excessive respiratory secretions.10 The trachea divides into paired bronchi relatively cranially in most chelonians.12 Because of this, it is important to resist passing an endotracheal tube far into the lower respiratory tract. Doing so may result in intubating a single lung and lead to inconsistent anesthesia.
Chelonian lungs occupy a large volume in the dorsal half of the coelomic cavity. The borders of the lungs include the periosteum of the carapace dorsally, the pectoral girdle cranially, and the pelvic girdle caudally.3 The lungs are multicameral, with a single intrapulmonary bronchus that divides into a complicated network of bronchioles and faveoli.13 Chelonian faveoli differ from mammalian alveoli in that they are compartmentalized, and oxygen exchange occurs on the reticulated surface of these compartments.2 The lungs are separated from the other coelomic viscera by a thin, nonmuscular, postpulmonary septum or pleuroperitoneal membrane (also called the septum horizonatale or the pseudodiaphragm).2,13,14 Chelonians do not have a true diaphragm and are therefore not dependent on negative pressure for breathing. The volume of the lungs is reduced to one fifth when the head and limbs are retracted.15
VENTILATION
To compensate for the absence of an expandable chest, chelonians have developed strong trunk muscles that help to expand and contract the lungs with active inspiration and expiration.15–17 It is the movement of the septum horizontale, powered by the movements of the trunk muscles, the viscera, and the limb girdles, that alters the intrapulmonary pressure and effectively draws air in and out of the lungs.2,3 When the septum horizontale is pulled taut and downward by the trunk muscles (e.g., limb movement), the area occupied by the lungs increases. This increase in lung volume thereby facilitates inspiration.2 For terrestrial chelonians, inspiration is passive and expiration is active, but this scenario is reversed for aquatic chelonians due to the effect of hydrostatic pressure on visceral volume.18,19
SUPPLEMENTAL RESPIRATORY ORGANS AND METHODS
Selected semiaquatic freshwater turtles possess the ability to absorb oxygen via the presence of well-vascularized cloacal bursae. This mechanism of oxygen absorption is utilized principally during periods of underwater hibernation. Some soft-shelled turtles can remain submerged for hours in the mud, utilizing oxygen in the water via exchange through the plastron and carapace, the villiform oral papilla, and the pharyngeal mucosa.3,20 Frye suggests that chelonians possess methods of glycolytic respirations, based upon the discovery that these animals can survive up to 8 hours in pure atmospheric nitrogen.12
Gastrointestinal System
UPPER DIGESTIVE TRACT
The chelonian oral cavity does not contain any teeth. Rather, chelonians use a sharp horny beak, or tomia, to grasp and tear food. The salivary glands of chelonians do not secrete digestive enzymes, but the stomach, small intestine, pancreas, liver, and gall bladder produce these proteolytic substances. The esophagus travels down the left side of the neck and terminates in the stomach, which is embedded in the left lobe of the liver.3
LOWER DIGESTIVE TRACT
The simple chelonian stomach is the proximal boundary of the lower digestive tract. The small intestine of chelonians is not well divided into a duodenum, jejunum, or ileum, and the tract is located in the caudal coelomic cavity.21 The mucosa of the small intestine is composed of single columnar epithelium,2 and regeneration of the intestinal epithelium takes an estimated 8 weeks in Chrysemys picta at a temperature of 20° C to 24° C (68° F to 75° F).22
The large intestine begins with the cecum, which is not a distinct organ but is rather a widening of the distal colonic wall.23,24 The cecum is located in the right caudal quadrant of the coelomic cavity. The large intestine is typically divided into ascending, transverse, and descending portions. The mucosal epithelium is comprised of a large number of glandular cells.2 Passage of food through the chelonian digestive tract is quite slow when compared to mammalian transit times (up to 2-4 weeks), but this allows for maximal nutritional absorption.25
The distal boundary of both the lower digestive tract and the urogenital tract is the cloaca. The cloaca is subdivided into three sections: the coprodeum, urodeum, and proctodeum. Of all the reptiles, subdivision of the cloaca is the least prominent in chelonians. The colon terminates at the coprodeum, and the coprodeum is separated from the more cranial urodeum by a distinct fold of tissue. The proctodeum is the most caudal aspect of the cloaca.2
LIVER
The chelonian liver is separated into indistinct lobes, and a small gall bladder is located at the caudal border of the right side. The liver functions basically in the same manner as the liver of other vertebrates, participating in lipid, glycogen, and protein metabolism. It is believed that the chelonian liver plays a pivotal role in the activation of calcitriol (vitamin D). It is important to note that the functionality and appearance of the chelonian liver is affected by both hibernation—when large amounts of fat may be deposited in the liver and decreased synthesis, storage, and release of liver products occur—and, in females, by reproduction—when vitellogenesis and increased protein synthesis occur. Primary hepatic pathology must therefore be differentiated from normal reproductive and seasonal hepatic variation.2
PANCREAS
As in other vertebrates, the chelonian pancreas is located adjacent to the proximal segment of the duodenum. The exocrine and endocrine physiologic functions of the chelonian pancreas are similar to that of higher vertebrates. Pancreatic secretions include a variety of different digestive enzymes, including amylase, trypsin, chymotrypsin, carboxypeptidase, elastase, lipase, ribonuclease, and chitinase. The other major pancreatic product is an alkaline secretion that neutralizes the acidic gut contents and creates a more suitable environment for the digestive enzymes.2
DIGESTIVE PHYSIOLOGY
Chelonian digestion is dependent on environmental temperature. More specifically, the amount and activity of secreted enzymes26,27 and the absorptive capability of the intestinal mucosa are directly related to temperature.21 Maximum digestion occurs within the animal’s appropriate temperature range (ATR), and the degree of digestion decreases when either above or below the ATR.2 At high temperatures, gastric hydrochloric acid production is reduced, thereby altering the pH of the stomach. Consequently, pepsinogen activity is reduced and digestion is slowed.2 Cell membrane permeability of the gut mucosa is also altered at different temperatures, thereby changing the absorptive processes of glucose and amino acids and the substrate affinity to trypsin.21 Seasonal fluctuations in digestive rates of chelonians also exist, with rates in summer being higher than those in spring.27
It has been determined that extremely slow digestion occurs between 10° C and 15° C (50° F to 59° F) and absolutely no digestion occurs below 7° C (45° F).21 When temperatures are below this critical level, putrefaction of the ingesta occurs. This occurs often in captive, hibernating chelonians and in captive, imported chelonians that rely on supplemental heat to maintain normal physiologic activities. The absorption of toxic putrefaction products may affect the normal functioning of the nervous or other body systems.2 Chelonian gastrointestinal contractions have been found to be temperature dependent as well.2 Chelonian gut contractions are not continuous but rather occur in a series.28,29 Gastric contractions begin at the cardia, run down the gastric wall in intervals of 21 to 31 seconds, and then terminate at the pylorus. These gastrointestinal contractions are controlled via vagal impulses, and the process is temperature dependent.30 Contractions of the small intestine occur at intervals of approximately 45 seconds.31 The first type of large intestinal peristalsis begins at the cecum and ends at the coprodeum. The speed of this type of contraction ranges from 0.15 to 0.5 mm/sec and typically results in defecation. The second type of contraction is an antiperistalsis, which starts at the coprodeum and pushes ingesta cranially for 2 to 3 cm. This type of antiperistalsis occurs at intervals of 18 to 25 seconds and allows urine to be shunted into the caudal parts of the colon where water and ions can be reabsorbed.21 Gastrointestinal transit time appears to be shortest in omnivorous species and longest in herbivorous species.21 The large intestines of herbivorous species tend to have a greater volume than those of omnivorous species.31
Renal System
Chelonians have two large, flat, lobulated kidneys that are located in the retrocoleomic cavity, ventral to the caudal lung fields and cranial to the pelvic girdle. The chelonian kidney lacks both a loop of Henle and a renal pelvis.2 The renal nephron is comprised of a glomerulus, a proximal tubule, an intermediate segment, and a distal tubule.32 The chelonian kidney produces urine that is hypotonic to isotonic to blood and actively excretes uric acid. Bilateral ureters enter the urodeum of the cloaca at approximately the 10 and 2 o’clock positions. As stated previously, antiperistalsis of the urodeum allows urine to be transported caudally into the coprodeum and colon to maximize water and ion absorption. Urine may also travel cranially from the urodeum to the bladder for water reabsorption. The bladder wall is lined with ciliated cells and secretes mucus. The urethra is relatively short in chelonians and enters the bladder through the midventral floor of the urodeum.2
RENAL PHYSIOLOGY
The chelonian kidney serves many important functions, including osmoregulation, fluid balance regulation, excretion of metabolic waste products, and the production of various hormones and vitamin D metabolites.31,33–36 Research has shown that the urine in the renal tubules and ureters of Gopherus agassizii is always hypo-osmotic to blood until passage through the urinary bladder, where it becomes isosmotic via presumptive electrolyte and fluid exchange.34
Urinary nitrogen is excreted in chelonians as a balance of ammonia, urea, uric acid, amino acids, allantoin, guanine, xanthine, and creatinine.32–3437 There are four described chelonian excretion patterns, each largely reflecting the environment in which an animal lives. Uricotelic and ureo-uricotelic chelonians tend to live in arid or desert environments, where water must be conserved. Uricotelic chelonians excrete predominately uric acid and urates, whereas ureo-uricotelic chelonians produce uric acid and urea as their major urinary excretory products. These chelonians are able to conserve water because uric acid is poorly soluble and can be excreted with minimal associated water.2 In uricotelic chelonians, hypo-osmotic tubular and ureteral urine is thought to decrease urate precipitation within the renal tubules during periods of dehydration.35 With uricotelism, urates precipitate out of solution in the bladder and do not need to be actively reabsorbed. Ureotelic and amino-ureotelic chelonians live in environments where water is plentiful. Ureotelism occurs when the major urinary excretory product is urea, whereas amino-ureotelism occurs when the major urinary excretory products are both ammonia and urea. Urea is highly water soluble and difficult to concentrate; therefore, it must be excreted with significant amounts of water.2
Ureteral urine has three possible pathways to follow once exiting the ureters, and the pathway decided upon is influenced by hydration status, fluid intake, and other biochemical factors. Urine may be directed into the coprodeum/proctodeum upon exiting the ureters. Some fluid reabsorption may occur in the colon or proctodeum, and the urine ultimately mixes with fecal material and is voided simultaneously. Urine may also be directed into the bladder, where electrolyte and fluid exchange occurs and isotonicity, regarding osmolarity and electrolyte composition, results. The bladder of uricotelic and amino-ureotelic species plays a significant role in fluid and electrolyte homeostatic regulation. Uric acid is precipitated out of solution, mainly as potassium urate, within the bladder and is consequently voided at urination. Finally, some ureteral urine may be excreted with no alterations to the fluid or electrolyte composition. It has been suggested that tortoise urination strongly coincides with drinking and bathing, and this is suggestive of an evolutionary mechanism that was developed to ensure the conservation of water in arid environments.2
Reproductive System
GENDER IDENTIFICATION
The majority of chelonians are sexually dimorphic, though differences are often not apparent in sexually immature juveniles. There are a number of ways to identify the gender of a chelonian. Male chelonians have a large, fleshy penis that is often heavily pigmented. The chelonian penis also has a median groove to assist in directing sperm during copulation. It must be remembered that clitoral hyperplasia, which may resemble a penis, has also been documented in chelonians. This typically occurs in females treated with oxytocin during dystocia and in debilitated, hypocalcemic, or edematous females. Males typically have longer, broader tails than females, and the distance from the caudal edge of the plastron to the cloacal opening is also generally longer in males than females (Figure 9-6).2
The male plastron of some terrestrial species is concave or indented compared to a female’s (Figure 9-7). This is thought to be an adaptation to assist with mounting. Adult males are frequently smaller than females of the same age. Male gopher tortoises (Gopher polyphemus) develop large mental glands in their mandibles. Red-eared sliders (Trachemys scripta elegans) develop long claws on their front legs and use them to visually stimulate the female turtle. Male box turtles (Terrapene carolina) develop red-colored irises, whereas females have yellow-colored irises. The gender determination of hatchling sea turtles has been described using testosterone levels of residual egg fluid.38 Environmentally sex determined species can potentially be sexed by monitoring the temperature of incubation.2 Oxygen concentration during incubation is also believed to affect the sex ratio of a clutch.39 Endoscopy is a useful, yet more invasive, technique to determine the gender of a chelonian.
MATING
In most terrestrial species of chelonians, the male mounts the female from above and behind, and the engorged penis is inserted into the female cloaca. Aquatic species mate underwater. Male courtship behavior, which oftentimes precedes mating, frequently includes characteristic vocalizations and aggression. Males of some chelonian species repeatedly ram females with their gular scutes or bite the head and limbs of trapped females. This behavior is considered necessary to induce females to be submissive for mating. The aggressive behavior, along with pheromones and mating itself, may also induce ovulation in receptive females. Not all ovulation is induced by mating, as evidenced by the fact that some female chelonians may store sperm for up to 4 to 6 years.2
REPRODUCTIVE PHYSIOLOGY
Females of most chelonian species exhibit annual reproductive cycles, but some species breed only every 3 to 4 years.40 Temperate chelonians tend to breed seasonally, whereas tropical species continuously breed.41 Reproductive physiology is influenced by many different environmental factors, including rainfall, humidity, food availability, presence of a suitable mate, and photoperiod.2
Two classes of chelonian vitellogenesis have been described. In the first type, vitellogenesis and follicular growth typically begin in late summer or autumn and are completed just before the winter hibernation. In the second class, which occurs in nonhibernating tropical chelonians, slow follicular growth occurs continuously but is not completed until just before ovulation. Ovulation typically occurs in the spring, and follicular development occurs directly before ovulation. The second type of vitellogenesis occurs less commonly.42 Some species of chelonians can exhibit both types of vitellogenesis, depending on the environmental conditions. Research in the area of reproduction suggests that some chelonian species may be induced ovulators.43 Ovulation may be influenced by pheromones, male courtship behavior (e.g., butting, biting, vocalizations), or the act of mating itself.
In chelonians, a distinct corpus luteum is formed following ovulation, and evidence suggests that both the corpus luteum and the chelonian ovary secrete progesterone.44,45 Progesterone has been shown to inhibit ovulation completely and to reduce oviduct and follicle size in Chrysemys picta.46 Estrogen stimulates vitellogenesis, the production of lipophosphoproteins by the liver, and their incorporation into the egg.41,45,47,48 It is thought that both estrogens and progesterone influence the hypothalamic release of gonadotropins.44
As stated previously, females of some species are capable of storing sperm for 4 to 6 years. Consequently, eggs from females obtained from the wild within this time frame should be considered possibly viable and worthy of incubation.12,49 Stored sperm is thought to reside in the narrow tubules of the albumin-secreting region of the oviducts,50 and internal fertilization is thought to occur within the oviduct, postovulation.12 Fertilized ova continue down the paired oviducts, and the cranial and middle portions of this structure function to produce both yolk and egg components. Calcification of the egg occurs in the caudal portion of the oviduct.12
Nesting behavior in chelonians includes a reduction in food ingestion, increased territorial behavior, climbing, and perimeter walking or pacing.39 Dystocias in captive chelonians can often be attributed to failure to provide a suitable nesting site with an adequate substrate at an appropriate temperature.51 During oviposition, most chelonians build a nest site via excavation with their hindlimbs. Clutch size varies depending on the species of chelonians, and multiple clutches are possible within one breeding season.
As stated previously, incubation temperature can play a significant role in gender determination in some chelonian species. Research in the area of reproduction of the leatherback turtle (Dermochelys coriacea) has revealed a proposed mechanism behind environmental sex determination. It is hypothesized that when eggs are incubated at higher temperatures, the enzyme aromatase is activated and this activation converts available androgen steroid substrate to estrogens. Without the activation of aromatase, no estrogen will be produced and the embryo will remain male.52,53
EGG DEVELOPMENT AND ANATOMY
Chelonian eggs are generally deposited in a protected environment and left to develop and incubate in the absence of maternal care. The shell of the egg is comprised of an external mineral layer and an internal fibrous layer. Soon after oviposition, the embryo begins to rise to the dorsal portion of the egg and the yolk settles to the ventral aspect.54 The first extraembryonic membrane to form is the vitelline or yolk sac membrane. This membrane eventually becomes vascularized and provides the early nutritional and respiratory needs of the embryo. Later in development, the amnion, chorion, and allantois form. The innermost membrane, the amnion, surrounds the embryo and provides fluid support. The allantois acts as a nitrogenous waste container and eventually fuses with the outermost membrane, the chorion. The chorion serves as the major gas exchange organ for the embryo.2 The development of an air space is variable among chelonian species, along with its location. The importance of this structure is unknown at this time but may play a role in gas exchange before pipping.55
The full-term embryo uses the egg tooth, or caruncle, to “pip” its way out of the egg. The extraembryonic membranes are eventually absorbed by the embryo, and after a variable amount of time, the hatchling leaves the egg.2
Nervous System
Chelonians have 12 cranial nerves.56,57 The brain is comprised of approximately 1% of the body mass and contains fairly well-developed optic lobes. The spinal cord travels to the tail tip and no cauda equina is present.57 A true arachnoid space is not present, and the area between the leptomeninges and the dura mater is known as the subdural space.3
Musculoskeletal System
The chelonian beak consists of an upper keratinized horny beak, known as the rhamphotheca, which overlies the maxilla. The horny beak attaches to the mandibular ramus at the dentary and contains no dentition.2 Unlike in mammals, the chelonian skull is devoid of articulations, except at the jaw.58 The skull also lacks true temporal openings. On either side of the bony skull, chelonians have large supratemporal fossae. The retractor muscles extend from the fossae and the supraoccipital crest and attach to the base of the skull, thereby allowing chelonians to retract their heads into their shells. The adductor muscles of the chelonian jaw run through a trochlear pulley system, which increases the length of the muscle fibers and provides extra strength. The pulley is formed by a process on the pterygoid bones in Pleurodira and by the quadrate bone in Cryptodira. The pulley system redirects the adductor muscle fibers in a vertical manner for maximum force, thereby allowing the skull of chelonians to remain small yet retain a strong bite.17,25 Chelonians open their beaks by lowering the mandible.3
The thoracic and lumbar ribs are integrated into the shell, and no sternum is present.2 There are 8 cervical and 10 trunk vertebrae, and the 10 ribs attach to the trunk vertebrae. Whereas the trunk vertebrae are fused together, the cervical vertebrae remain independent of one another, allowing for the bending of the neck sideways (Pleurodira) or retraction into the shell (Cryptodira).3 Modified thoracic and pelvic limb girdles are present inside the ribs and are fused to the carapace in Pleurodira but not in Cryptodira.3 The pectoral girdle consists of the epiplastron (clavicle), the entoplastron (interclavicle), the scapula, the acromion process, and the coracoid bone. The pelvic girdle is comprised of the ilium, ischium, and pubic bones, all of which are paired and meet at the acetabula. The ilium is attached dorsally to the sacral ribs.3 Chelonians generally have pentadactyl limbs that extend more laterally than mammalian limbs. The humerus and femur are short in length, and the carpus and tarsus are both fused. Chelonians have five claws on each foot, with the exception of tortoises—which have short stubby toes and only four claws on the hind feet—and three-toed box turtles (Terrapene carolina).3
Sensory System
SIGHT
Vision is highly developed in chelonians.3 Chelonian eyes are located within orbits in the bony skull and are separated medially by bony structures. In some species, the septum is partially fibrous. A nictitans membrane is present in the majority of chelonian species. The muscles controlling eye movements are the same as those in mammals and consist of the superior and inferior obliques, anterior and posterior retractor bulbi, levator bulbi, and superior and inferior optic rectus muscles. The Harderian gland produces an aqueous secretion and lies in the nasal area. The gland opens into the ventral conjunctival sac, and the ducts of the Harderian gland drain into the craniomedial angle of the eye between the membrane nictitans and the conjunctiva bulbi. The lacrimal gland also produces an aqueous secretion and opens into the conjunctival sac. It is located in the temporal region of the orbit.2 Due to the lack of nasolacrimal ducts, tears are removed via simple overflow, evaporation, and absorption by the conjunctival mucosa.59 Evaluation of tear production via the Schirmer tear test is not useful in chelonians.
The cornea of chelonians lacks a Bowman’s layer, which is present in the cornea of higher vertebrates. The epithelium of the cornea is very thick and is lined by a thin Descemet’s membrane. The iridocorneal angle is less developed than in mammals, and the shape of the anterior chamber is maintained via the production of aqueous humor. Research conducted by Selmi et al. determined that the right and left intraocular pressures of red-footed tortoises (Geochelone carbonaria) were 14.5 ± 8.2 and 15.7 ± 9.3 mmHg, respectively. No change in intraocular pressure was noted as the animals aged.60 As in birds, the iris sphincter of chelonians is under voluntary control.
The chelonian retina is avascular. Nutrition is provided by choroidal vessels and a vascular projection into the vitreous from the optic nerve head, the conus papillaris, as in the avian pectin. The retina also contains more cones than rods, allowing chelonians to discern color differences. Many North American box turtle (Terrapene) species are attracted to red and orange,61 whereas Testudo species prefer red and/or yellow.2 Herbivorous species appear to recognize the color green. Species that have evolved in low light intensity environments typically have larger globes relative to the overall head size. This grants these species a greater ability to see in low light intensity areas.2
OLFACTION
Chelonians have a well-developed olfactory system, and extensive and highly refined chemosensory cells are located within the nasal epithelium.2 The Jacobson’s organ is an accessory olfaction organ that all reptiles possess. It is a paired organ that is located on the rostral roof of the oral cavity over the vomer bones. The Jacobson’s organ is simply a localized area of sensory epithelium that is innervated by the vomeronasal nerve (a branch of the olfactory nerve) and is less developed in chelonians than other reptiles.3 Gular movements in semiaquatic species, which occur when water is pumped through the nasal chamber by pharyngeal contractions, are believed to be related to the olfactory system rather than the respiratory system. Taste buds are also located throughout the oral epithelium, and both taste and smell appear to play a role in appetite stimulation and normal feeding.2
HEARING
Chelonians do not have a well-developed sense of hearing. Chelonians do not have external ears but rather exhibit the tympanic membrane (a layer of simple undifferentiated skin). The tympanic membrane lies caudal to the eye, at the level of the commissure of the beak. The function of the tympanic membrane is to protect the middle ear cavity. The tympanic cavity is separated by a process of the quadrate bone into a lateral tympanic cavity and a medial recessus cavi tympani. The inner ear is protected by this bony process. Sound is transferred from the tympanic membrane to the underlying extracolumella, a cartilaginous disc that is connected to an ossicular process, known as the columella. The columella is made up of a thin osseous shaft that penetrates through a small hole in the bony ventral process of the quadrate bone to contact the inner ear. The inner ear then ends in a funnel-shaped plate. Rotational movement is also transferred to the inner ear in the same manner, and it is believed that chelonian ears may be more important for balance than for hearing. It is thought that chelonians are only capable of hearing low tones, which may be due more to vibrations than to auditory signals.2
HUSBANDRY
Environmental Conditions
ENCLOSURE SIZE
The shape and size of the enclosure should be selected based on the chelonian’s habitat preference. Aquatic species should be provided deep, leak-proof enclosures, whereas tortoises can be provided shallow containers. All chelonians should be provided the largest enclosure possible. Some recommendations call for the enclosure to be at least four times the size of the turtle’s carapace; however, we feel this is too small. Instead, we prefer that the enclosure be at least 10 times the carapace diameter. Maximizing the surface area of the enclosure is important for ensuring ample area for exercise and an appropriate thermal gradient. Providing a large enclosure is also important because of the feeding habits of the chelonians. Tortoises are grazers and can generally consume large quantities of grass hay in their enclosure. If the enclosure is too small, the animals will overconsume their substrate. For aquatic animals it is important because these animal consume their food in the water. For those species that require live food, a larger enclosure ensures that the animal must exercise, providing enrichment, to capture its prey. A larger enclosure also helps to ensure better water quality, as small enclosures can become fouled relatively quickly.
Chelonian enclosures can be made from a variety of materials. The most common enclosure for aquatic species remains the glass fish tank. These enclosures are relatively inexpensive, easy to clean, and provide direct visualization of the pet. These are not considered as ideal for tortoises because they are poorly ventilated; however, limited ventilation can also be a problem for aquatic species. Plastic storage boxes are another common commercial product used to house chelonians. These enclosures are also inexpensive, easy to clean, and readily available. Many of these enclosures are not clear, so direct visualization may be limited; however, with chelonians the risk of escape is minimal so lids are not generally necessary unless they are being used to prevent unauthorized contact with children or domestic pets. Tortoises do not generally fare well in wire-based cages, as they may develop rostral or limb abrasions while trying to pass through the cage. We have found that tortoises can be kept outdoors in pens created from wood or tin (Figure 9-8). Any tortoises or turtles kept in outdoor pens should be protected from both domestic pets (e.g., dogs) and wildlife predators.
HUMIDITY
Humidity is an important environmental factor to consider for tortoises, but it is less important for aquatic species because they live in a moist environment. In general, desert tortoise species tolerate humidity levels between 30% and 50%, subtropical species from 60% to 80%, and tropical species 80% to 90%. Chelonians maintained in environments with low humidity may become anorectic and dehydrated and develop reduced gastrointestinal transit times. Excessive humidity is frequently associated with the development of dermatitis and respiratory disease. Humidity levels can be monitored using a hygrometer. Digital hygrometers are preferred because they can be moved around within an enclosure to determine areas that are appropriate and inappropriate. Humidity levels can be altered in an enclosure by adding a large water bowl, aerating water within a water receptacle (e.g., bowl or mason jar), adding an incubation chamber, misting the enclosure daily, or adding live plants. Large water bowls serve as a source of humidity for an enclosure, as the water evaporates into the enclosure. The overall contribution of moisture to the humidity can vary based on the environmental temperature and size of the enclosure. Daily misting is a simple method for elevating the humidity but may provide only a temporary increase to the humidity.
LIGHTING
Historically, lighting systems for captive chelonians have not been given a great deal of attention. Herpetoculturists who maintain their animals indoors often provide only ambient light. Animals maintained outdoors derive the most benefit from routine exposure to direct or indirect sunlight. A recent study in red-eared sliders (Trachemys scripta elegans) found that animals provided exposure to full-spectrum fluorescent lighting were significantly more likely than controls to have higher 25-hydroxyvitamin D plasma levels.62 Full-spectrum lighting has many benefits, from the provision of ultraviolet radiation to rich, visible light. Although the benefits of full-spectrum lighting have not been evaluated in other species of chelonians, we recommend providing captive chelonians full-spectrum lighting to mimic the beneficial effects of sunlight. Chelonians should be provided a 12-hour photoperiod. The photoperiod can be altered for the breeding season (lengthened) or brumation period (shortened).
SUBSTRATE
Substrate selection for chelonians is an important consideration, as many of these animal are geophagic and can develop foreign bodies if provided an inappropriate substrate. Aquatic species should be provided a gravel substrate. Larger pieces of gravel that are not edible are preferred. Sand may be used but is generally more difficult to manage, is easily stirred, and may increase the water turbidity. Aquatic species not provided a substrate may develop pressure sores, especially if maintained in plastic enclosures. Terrestrial species may be housed on a variety of different substrates. When considering which substrate is best for a particular species, it is important to attempt to replicate its natural habitat. Most of these animals are naturally found on grass, detritus, or rock-based substrates. Maintaining tortoises in outdoor facilities, when weather permits, provides the most realistic substrate. This type of substrate also provides a regular food source. The provision of a grass substrate is more difficult for captive, indoor tortoises. The following is a list of common substrates used for tortoises held indoors: newspaper, paper towels, alfalfa pellets (Figure 9-9), large gravel, orchid bark chips, grass hay, and straw. Newspaper and paper towels are commonly used for juvenile animals and recently acquired animals because they are inexpensive, can be easily replaced, and allow a veterinarian or herpetoculturist to monitor fecal and urine output. There is a wives’ tale that the ink found on newspaper is potentially toxic to animals, but this belief is unfounded. Gravel can be used but should besufficiently large enough to prevent ingestion. Orchid bark chips can be used as a substrate for those species naturally found in forested areas. Natural leaf litter can also be used but should be thoroughly inspected for the presence of pest bugs before being placed into the vivarium. Alfalfa pellets and grass hay are commonly used because they can serve as both a substrate and source of nutrition. However, these substrates are easily soiled and can serve as a source of infection (e.g., bacterial or fungal) to these animals. In any case, the type of substrate to be used should be selected based on the specific needs of a particular animal. If one substrate does not appear to be of value, another should be evaluated.
WATER QUALITY
Aquatic species must be provided access to a clean water source. The best method for ensuring that the animal’s aquatic environment is clean is to use filtration. Many of the filtration methods used to maintain aquatic systems for ornamental fish can be used for turtles, although turtles can produce significantly more biologic waste than ornamental fish. The primary filtration units used for aquatic systems are mechanical, chemical, and biologic. Mechanical filtration consists primarily of material that traps organic material. Many of the new power or canister filters use sponge-like materials and floss as mechanical filters. Chemical filtration is generally done using the addition of specific chemicals (e.g., those that chelate ammonia) or charcoal. Charcoal binds to noxious chemicals, rendering them nontoxic. Ultraviolet radiation can also be used as a chemical filter to kill potential pathogens. Biologic filters are based on the premise that living bacteria naturally degrade potential toxins (e.g., ammonia and nitrite) to less toxic compounds (e.g., nitrate). These filters work by providing surface area within the enclosure for the bacteria (e.g., Nitrosomonas spp. and Nitrobacter spp.) to colonize. All three of these filtration methods can be used in aquatic turtle systems. It is important to recognize that because turtles produce significant quantities of organic waste, mechanical filters must be changed more regularly than in ornamental fish systems.
NUTRITION
Dietary Requirements
Chelonians may be herbivorous, omnivorous, or carnivorous, depending on the species. An ideal diet for captive chelonians mimics their natural diet as closely as possible and provides a diversified selection of food. Some herbivorous species will often readily eat an omnivorous diet,63 but eventually these animals will reveal signs of nutritional deficiencies.64 Therefore, it is important to remember that food preferences do not always correlate with appropriate nutrition.
Herbivorous Chelonians
Herbivorous tortoises and marine turtles are classified as hindgut fermenters,65,66 with microbial fermentation occurring in the large intestine. Consequently, the bulk of the diet of herbivorous tortoises should be vegetable fiber. The vegetable fiber offered to tortoises should be rich in vitamins A and D3 and should have more available calcium than phosphorous.39 An ideal Ca:P ratio of at least 1.5 : 1 to 2 : 1 should be present.67 The diet should also be low in fats, oils, proteins, thiocyanates, and oxalates.39 In captivity, tortoises are typically fed weeds, flowers, and grasses on a daily basis. Tortoises will forage for themselves if provided an appropriately planted enclosure, but additional food is usually required. It is important to periodically peruse the yard and rule out the presence of any poisonous plants, such as rhubarb,63 daffodils, potatoes, buttercup, and yew. A variety of foods should be offered and can be mixed with calcium, iodine, vitamin D3, and vitamin A supplementation. It is important to remember that grocery greens are generally higher in protein and lower in fiber and may have an inverse calcium-to-phosphorus ratio to natural forage.68 Spinach, cabbage, and beet greens should not be fed in excess due to their high oxalate content. The majority of foods designed for dogs, cats, humans, and other mammals should not be fed to tortoises. Debilitated tortoises requiring force-feeding or tube-feedings should be fed a critical care diet designed for herbivorous reptiles.
Omnivorous Chelonians
It has been suggested that omnivorous chelonians do best when offered plant and animal matter in proportions that range from 75 : 25 to 90 : 10.69 Dietary requirements in these species tend to change with age, with most juveniles requiring a diet comprised of a higher proportion of animal matter. As the juveniles mature, their dietary requirements shift to a more herbivorous diet.68
Free-ranging omnivorous chelonians will ingest earthworms, slugs, snails, millipedes, pupae, and maggots. It is essential to monitor the diets of captive invertebrates to avoid nutritional deficiencies in the chelonians that are ingesting them. The insects, worms, and pupae should be offered a diet rich in minerals and vitamins to ensure that the prey is “gut-loaded” and will meet the nutritional needs of the predator species. The invertebrate prey should also be dusted with calcium powder before feeding to ensure that the proper calcium-to-phosphorus ratio is maintained.63 Chelonians appear to prefer “slightly rotten” fruits and vegetables, and their diets may be occasionally supplemented in very small amounts with meat, fish, or low-fat dog or cat food.68 However, it is important to consider that “slightly rotten” foods may have more microbes associated with them and may predispose the chelonian to food poisoning. In addition, mammalian diets should generally be avoided long term as they may be too potent (e.g., excess protein and vitamins) for a chelonian. Liver and yellow or dark orange colored vegetables (squash, carrots, sweet potatoes) are excellent sources of vitamin A,70 and Swiss chard, kale, beet greens, escarole, parsley, watercress, and green beans all have a positive Ca:P ratio.
Most semiaquatic chelonians are omnivorous. There are many commercial pelleted diets available (e.g., Aquatic Turtle Diet, Fluker Farms, Port Allen, LA) that suit the nutritional needs of these particular chelonians (Figure 9-10). Some species, such as the Chinese three-striped box turtle (Cuora trifasciata) and the Malayan box turtle (Cuora amboinesis), prefer to be fed in the water. Abnormal shell and bone growth may occur if the diet being offered is too high in animal protein. These diets are often also high in phosphorus, which disrupts the normal calcium-to-phosphorus ratio. Fruits and vegetables should be dusted with calcium and vitamin powder just before being offered. Obesity can be avoided by feeding adults three to four times per week.68

Figure 9-10 Commercial turtle chows can be used in combination with natural food items to diversify an animal’s diet.
Asian box turtles (Cuora spp.) are a commonplace captive semiaquatic chelonian. Hatchlings feed almost entirely on animal prey, whereas adult turtles require vegetable and fruit supplementation. It has been suggested that one third to one half of the diet offered to adults should be plant based, and the remaining one half to two thirds should be animal matter. The plant portion of the diet should be made up of 70% to 80% vegetable matter and 20% to 30% fruit matter.70 Plant matter offered should include collard and mustard greens, kale, bok choy, dandelions, apples, grapes, strawberries, bananas, and pears. Animal matter suitable for ingestion includes whole, skinned, chopped mice; pinkies; trout chow; earthworms; crickets; slugs; maggots; and sardines.68
The red-eared slider (T. scripta elegans) also feeds on a variety of animal and vegetable matter and almost exclusively feeds in the water. Greens, fruit, commercial turtle chow, pond fish pellets, raw whole fish, liver, and insects are commonly offered.68 The adult red-eared slider is primarily herbivorous.68
RESTRAINT
Manual Restraint
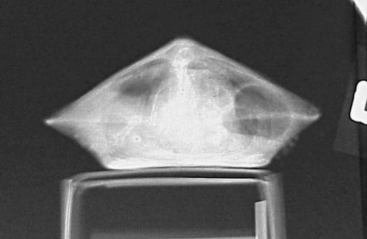
The chelonian patient rarely requires significant or forceful restraint; however, large freshwater snapping turtles and marine turtles may pose exceptions to this. Small chelonians can easily be handled with a hand on either side of the carapace (Figure 9-11). Larger chelonians may be restrained with the placement of one hand around the cranial carapace, dorsal to the neck, with the other hand around the caudal carapace, dorsal to the tail. The beak should always be directed away from the handler. To passively restrain a chelonian patient for radiographs or some other procedure, the patient may be placed on a raised object, such as a roll of tape or a circular feeding dish, which is positioned in the center of the plastron (Figure 9-12). With nothing for their limbs to make contact with, the patient is effectively immobilized. It is very important to note that hypothermia is an inappropriate method of restraint. The chelonian head can be restrained by grasping the base of the skull at the point of the mandibular rami with the index finger and thumb. Gentle traction should be used to extend the head and neck. Never use excessive pressure, as this can lead to cervical spinal injury.

Figure 9-11 For nonaggressive chelonians, the animal can be restrained by grasping the sides of the shell.
PERFORMING A PHYSICAL EXAMINATION
Head and Associated Structures
The position of the eyes in the sockets should also be closely examined. The eyes may appear bilaterally enophthalmic in cases of severe dehydration, whereas bilateral exophthalmia may be observed concurrently with generalized edema. Unilateral enophthalmia is suggestive of injury or trauma, and unilateral exophthalmia is consistent with either trauma or the presence of a retrobulbar abscess or tumor.71
Shell
The shell should be examined for scute quality. Pyramiding of the scutes has been associated with excessive dietary protein ingestion and limited access to water (Figure 9-13). Abnormal keratinization, ulceration of the shell, discharges, swelling, soft areas, and malodorousness should be recorded. Poor calcification of the shell is often associated with secondary nutritional hyperparathyroidism, and a reddish hue of the plastron may be suggestive of septicemia (e.g., vasculitis). Ulcerative shell disease is most consistent with the presence of shell ulceration and discharge.
Skin
The skin should be examined for abnormal shedding, sloughing, edema, ulceration, abscessation, or discharge. Decreased skin elasticity is often consistent with dehydration. Excessive administration of vitamin A may result in blistering and sloughing of the skin on the limbs (Figure 9-14). The exudate is often clear. The skin should also be examined for parasitic infestation. This is most commonly observed in chelonians recently captured from the wild.
Coelom
The coelom may be palpated via insertion of one to two fingers just in front of the caudal limbs in the prefemoral fossae (Figure 9-15). The presence of ascites, visceral enlargement, a space-occupying mass, or a calcified egg can be assessed via digital palpation. Calcified eggs can also sometimes be found inside the coprodeum via gentle palpation on either side of the tail.
Auscultation
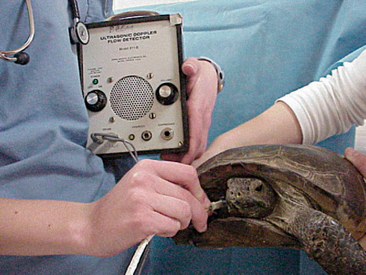
It is generally considered difficult to auscultate the heart and lungs of a chelonian using a standard stethoscope. We have found that a crystal ultrasound Doppler is the best method for assessing the heart. The Doppler probe should be placed between the head and forelimb (either side) and directed caudallytoward the heart (Figure 9-16). The same technique can be used to assess the heart during anesthesia.
DIAGNOSTIC TESTING
Hematology
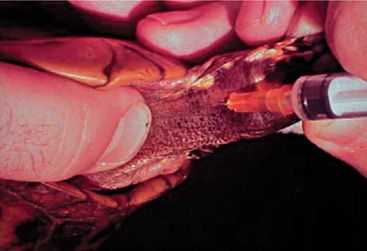
Blood can be extracted from the chelonian patient using a variety of techniques and venipuncture sites. A maximum of 5 to 8 ml/kg body weight of blood may be collected, but typically 1 to 1.5 ml/kg is sufficient. The jugular vein yields blood samples that are least likely to be lymph diluted and is the vessel of choice in patients weighing less than 4 kg. The head and neck may be extended and restrained via placement of a finger and thumb behind the occiput. The chelonian can then be placed in lateral recumbency, and the jugular vein can oftentimes be visualized as it extends caudally from the angle of the mandible. The vessel should be entered in a caudal-pointing direction (Figure 9-17). The dorsal venous sinus, or the dorsal coccygeal vein, may be utilized in some species of chelonians and is typically found in the dorsal midline of the tail. It must be mentioned that lymph dilution may occur in this site and potential damage to the central nervous system may ensue if the subarachnoid space is entered and cerebral spinal fluid is sampled without proper disinfection of the sampling site.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree