CHAPTER 7 SNAKES
COMMON SPECIES KEPT IN CAPTIVITY
Snakes are listed in the class Reptilia and order Squamata. Squamata include the suborders Serpentes (snakes) and Sauria (lizards). There are over 2900 species of snakes in the world. Extant populations of snakes are generally divided into three classes: Scolecophidia, Alethinophidia, and Caenophidia.1
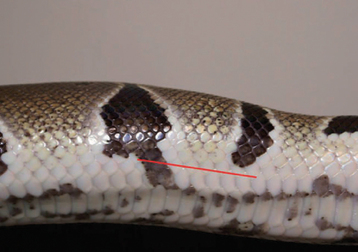
The corn snakes (Elaphe guttata) and king snakes (Lampropeltis spp.) are by far the most common snakes kept in captivity. Most corn snakes and king snakes attain a total length of 1 to 2 m (3-6 feet). These animals are known for their relatively calm dispositions, although some king snakes can be aggressive. Corn snakes make good first pet reptiles. Corn snakes and king snakes have been selectively bred over the past decades for their color morphs. A wide variety of color morphs have been developed from the common (e.g., amelanistic) to the uncommon (e.g., lavender) (Figure 7-1). The value of the snake can increase significantly based on its color. This is most evident when evaluating the other common snakes held in captivity, pythons and boids. Designer-colored ball pythons (Python regius) can range in price from the hundreds of dollars to tens of thousands of dollars (Figure 7-2). Pythons are popular not only for their beauty but also for their gentle disposition. There are certainly exceptions to this rule. The giant pythons, such as the Burmese (Python molurus bivittatus) and reticulated pythons (Python reticulatus), become too large for most pet owners and should not be recommended as pets (Figure 7-3). Instead, these animals should be handled only by those experienced herpetoculturists that accept the responsibility and liability that comes with giant snake ownership. Many state and local governments have established regulations banning large snakes. Veterinarians should become familiar with both state and local policies to ensure that they can make the most appropriate recommendations to their clients.
BIOLOGY: ANATOMY AND PHYSIOLOGY
Snake skin is relatively inelastic, as a result of the rigid protective scales (beta-keratin). This might be considered a problem for an animal that must swallow its prey whole, but is not as a result of a thin elastic alpha-keratin region. The alpha-keratin includes the interscalar region and is most obvious in snakes during the ingestion of prey or in obese animals as their skin stretches. Snake scales originate from the epidermis. The integument of the snake is covered with two primary scale types, both originating from the epidermis. Small scales cover the dorsum and lateral surfaces of the snake, whereas larger scales cover the ventrum. It is important to make a distinction between the two scale types when considering an exploratory coeliotomy, as an incision in the ventral scales could result in significant tension on the sutures after closure and an increased likelihood of the incision becoming contaminated. I prefer to make the initial incision between the second and third rows (from the ventrum) of dorsal scales (Figure 7-4). Differences in scale shape, size, and texture can be used to differentiate snake species. For example, the presence of keeled versus nonkeeled scales is a basic taxonomic key. The snake’s integument should feel dry and warm (if it is housed under appropriate environmental conditions), which is in direct contrast to the public’s perception that snakes are cold and slimy. Snake skin is dry because it is relatively aglandular, although some reptiles have developed regionalized glands that are useful in attracting mates or in defending themselves against predators.
Ecdysis (shedding) is a naturally occurring process in the snake that is regulated by the thyroid gland. Snakes should shed their entire skin, including their spectacles, in one piece, although large snakes (>3 m) may shed their skin in pieces. Once the process of ecdysis is started, it generally takes approximately 14 days to complete. During this period it is not uncommon for a snake to become anorectic, appear listless, and avoid contact. Immediately before ecdysis, some snakes have increased respiratory sounds. This is generally associated with reduced airflow through the nares, resulting from a constriction of the shed surrounding the nares. A small-gauge hypodermic needle (25-gauge) can be used to remove any obstructive material. Excessive handling during the ecdysis period is not recommended, as it is possible to damage the newly formed underlying epidermis. Approximately 14 days before the shed, the snake will have a dull (grayish) appearance. The spectacles take on a “blue” color 7 to 10 days before the shed and then clear 2 to 3 days later. The dull color is associated with the lymphatics and enzymes that fill the space between the old and new epidermal layers. Captive snakes should be provided appropriate cage furniture (e.g., rock) to rub their rostrum against to facilitate the shedding process.
The digestive tract of the snake is a linear system that is modified to digest dense meals. The tongue is an important chemosensory structure. The tongue is inserted into the Jacobson organ, located in the roof of the buccal cavity, to differentiate odors. Because snakes hunt by olfaction, they can be trained to readily accept prekilled prey items. Snake teeth are homodontic, with the exception of the fangs in venomous species. The teeth of snakes have developed with the sole purpose of acquiring prey. Their general shape, being curved backward, is based on their need to apprehend and hold prey in the absence of limbs. To remove a snake that has bitten someone, it is important to consider the direction of the teeth, as pulling the animal directly from the site where it is attached will tear the skin and worsen the bite wound. Instead, the animal’s head should be gently directed forward as it is being removed. Of course this is easier said than done in some cases. Snake teeth also are pleurodontic, and are therefore replaced throughout the animal’s life. Of all the reptiles, snakes by far have the most teeth, with rows on the upper and lower jaws and palatine bone. Mucus excreted from the digestive glands located in the buccal cavity and esophagus lubricate prey items to facilitate ingestion. Venom glands are modified salivary glands that produce potent enzymatic compounds to assist with prey acquisition and digestion. The esophagus is a large distensible organ in the snake. The stomach is linear and produces digestive enzymes. The stomach is located approximately half the body distance from the head. The small intestine and colon are also linear structures, losing much of the coiling frequently observed in omnivores and herbivores. The cloaca is comprised of the coprodeum, urodeum, and proctodeum. The coprodeum is located in the dorsal region of the cloaca and receives fecal material from the colon. The urodeum is located ventrally and serves as the collection site for urine from the ureters. The oviducts also open into the urodeum. The proctodeum is the collecting chamber for the urodeum and coprodeum.
HUSBANDRY
Environmental Conditions
ENCLOSURE SIZE
Snake enclosures can be made from a variety of materials. The most common remains the glass fish tank. These enclosures are relatively inexpensive, are easy to clean, and provide direct visualization of the pet. Plastic storage boxes are another common commercial product used to house snakes (Figure 7-5). These enclosures are also inexpensive, easy to clean, and readily available. Many of these enclosures are not clear, so direct visualization may be limited. Commercial snake enclosures have become quite popular (Figure 7-6). These enclosures are aesthetically pleasing and easy to clean. One disadvantage of commercial enclosures is that they are more expensive than traditional enclosures.
TEMPERATURE
Snakes are ectotherms and depend on the environmental temperature to regulate their core body temperature. If these animals are not provided an appropriate temperature range, their metabolic rate slows. Snakes with slow metabolic rates often have a history of being anorectic, lethargic, and depressed. An inability to maintain an appropriate body temperature can also result in a reduced immune response. In my experience, many of the snakes with chronic infections are not provided exposure to an appropriate environmental temperature. To prevent these problems, snakes should be provided an environmental temperature range. The environmental temperature should mimic the animal’s natural climate and should be separated into different gradients. It is important to realize that reptiles have temperature options in nature. They can bask under the sun on a rock if they wish or hide in the shade away from the heat to prevent overheating. This same system should be mimicked in captivity. I recommend that clients use variable wattage incandescent light to create a temperature gradient. Bulb wattage will depend on the size of the enclosure. A list of recommended environmental temperature zones for captive snakes can be found in Table 7-1. It is important to provide snakes a cooling period at night. For most of the temperate species, room temperature is acceptable, whereas for some tropical species, additional heat may be required. Visible light (e.g., incandescent bulbs) should not be used at night to provide heat. It is important that animals are provided a period of darkness. I generally recommend a photoperiod of 12 hours per day. This can be altered during the breeding cycle (lengthened) or aestivation period (shortened). When supplemental heat is required during periods of darkness, ceramic heat emitters or under-tank heating elements can be used.
TABLE 7-1 Environmental Temperature Range Recommendations for Captive Snakes
| Species | Daytime temperature range | Nighttime temperature range |
|---|---|---|
| Corn snake | 76–86° F (24–30° C) | 70–75° F (21–24° C) |
| California kingsnake | 78–88° F (25–30° C) | 70–78° F (21–25° C) |
| Ball python | 80–88° F (26–31° C) | 75–80° F (24–26° C) |
| Boa constrictor | 80–88° F (26–31° C) | 75–80° F (24–26° C) |
| Burmese python | 80–88° F (26–31° C) | 75–80° F (24–26° C) |
| Brazilian rainbow | 78–88° F (25–31° C) | 75–80° F (24–26° C) |
| Emerald tree boa | 80–88° F (26–31° C) | 75–80° F (24–26° C) |
| Milk snake | 76–86° F (24–30° C) | 70–75° F (21–24° C) |
Regardless of the heat source, snakes should not be allowed direct contact with the heating element. Animals that are allowed direct contact can develop severe thermal injuries (first to third degree). Thermal injuries should be managed aggressively. Necrotic tissue should be sharply dissected and the wound protected. I apply a hyperosmotic solution (50% dextrose) onto the wound as a microbicide. The hyperosmotic is left on the wound for 2 minutes and than rinsed away with a 0.9% saline. The fluids are always warmed (to the animal’s preferred optimum) before being applied. The application of cooled or room temperature fluids can lead to a lowering of the body temperature and a reduced healing time. The hyperosmotic is applied twice daily until sufficient granulation tissue appears. The wound must be protected against desiccation. Silver sulfadiazine can be applied to the lesion to prevent desiccation and provide an antimicrobial effect. This compound is comprised of both a microbicide (silver) and an antibiotic (sulfadiazine). Bandaging these wounds can be difficult. Frye2 has suggested using condoms, but even these can fail. I have used Tegaderm (3M, Inc., St. Paul, Minnesota) with some success. These wounds can require weeks to months to heal. Snakes with severe thermal burns should be provided fluids to replenish any losses incurred either directly as a re-sult of the injury or after the injury until the integument heals. Systemic antibiotics also should be considered in the treatment plan.
LIGHTING
Historically, lighting systems for captive snakes have not been given a great deal of attention. Many snake breeders maintain their snakes in stacks of enclosures, where the animals only receive indirect room lighting. Although many of these animals thrive and reproduce successfully, it is my belief that snakes, like other reptiles, should be provided high-quality, full-spectrum light. Full-spectrum light has many benefits, from the provision of ultraviolet radiation to rich, visible light. Recent work by my laboratory has found that certain snakes increase their 25 hydroxyvitamin D blood levels with exposure to ultraviolet B radiation (Figure 7-7). It is not known whether snakes require ultraviolet B radiation to initiate the synthesis of vitamin D. Many believe that these animals obtain their vitamin D through the ingestion of prey. Regardless, ultraviolet A and visible light remain important to snakes for regulating behavior and stimulating reproduction. I recommend providing captive snakes full-spectrum lighting to mimic the beneficial effects of sunlight. Snakes should be provided a 12-hour photoperiod. The photoperiod can be altered for the breeding season (lengthened) or brumation period (shortened). Research to elucidate the specific lighting needs of snakes should be pursued.
PREVENTIVE MEDICINE
Quarantine
The concept of quarantine is rather foreign to most herpetoculturists and veterinarians. Although they understand its value, it is often difficult to enforce. I recommend the following: All snakes should be quarantined for a minimum of 2 months before being introduced into a reptile collection. However, it is important to realize that even a 2-month quar antine may be insufficient time to detect certain viral, parasitic or bacterial diseases in which a snake is a latent carrier. A thorough physical examination and appropriate diagnostic screening assays should be performed before the snake is placed into quarantine and before removing the animal from quarantine. A complete blood count may be done to evaluate the animal’s white blood cell status. An inflammatory leukogram may be indicative of an infectious disease. Serologic testing for snakes is limited to paramyxovirus. This is certainly a virus that a herpetoculturist would not want to introduce to a collection.
RESTRAINT
Manual Restraint
Before restraining an animal, it is important to identify its “weapons.” In snakes, their primary weapon (defense) is their teeth. In some colubrids, boids, and pythons, their ability to constrict should also be considered a potential weapon. By effectively restraining the head and body of a snake, the veterinarian will remove the animal’s weapons. Nonvenomous snakes can be effectively restrained by grasping the head of the animal at the level of the quadrate or mandible with one hand and supporting the snake’s body with the other hand (Figure 7-8). An additional handler should be used for every 3 to 4 feet of snake to support the snake’s spine. Snakes should never be draped over the neck of an individual.
Venomous snakes should be handled only by trained professionals. Hooks and tongs should be used to remove venomous snakes from their enclosure. Once collected from an enclosure, the snake should be directed into an appropriately sized clear plastic tube. The snake should be allowed to crawl to the midpoint of the tube and then prevented from advancing (Figure 7-9). This technique allows for safe management of the snake and allows veterinarians to grossly examine the snake and collect blood from the ventral coccygeal vein. Intravenous (IV) propofol (5 mg/kg) can be administered slowly via the ventral coccygeal vein or isoflurane directed into the tube to anesthetize the snake.
Chemical Restraint
Most snakes can be safely restrained manually; however, in those cases where the snake poses a risk to the veterinarian or is too fractious for a possible procedure, chemical restraint should be considered. The dissociative agents (ketamine and tiletamine) are the preferred anesthetics for these cases. Ketamine (5-10 mg/kg intramuscular [IM]) or tiletamine (3-5 mg/kg IM) will provide sedation in most snakes. In my opinion, these doses are not appropriate for procedures that may induce pain. For more complete general anesthesia, a higher dose of these compounds may be necessary (10-20 mg/kg ketamine, 5-8 mg/kg tiletamine) or different compounds altogether (e.g., propofol or isoflurane) (see Anesthesia).
PERFORMING A PHYSICAL EXAMINATION
It is important to assess the cardiorespiratory organs of a snake. In the past this has not always been recommended, because assessing the heart and lungs of a snake using a stethoscope can be difficult. I have found that an ultrasonic crystal Doppler can be used consistently to determine the heart rate of an animal and evaluate the snake for cardiac irregularities (Figure 7-10).
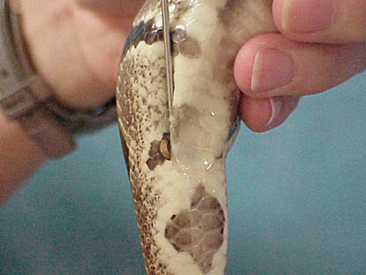
The gender of the snake should be determined as part of a routine examination. Some veterinarians charge an extra fee for determining the gender of a snake; some incorporate this into the physical examination. Knowledge of the gender of an animal is important when considering the signalment and potential differential diagnoses. Male snakes have two copulatory organs, the hemipenes. These structures are located in the base of the tail, caudal to the vent. The gender of a snake can be determined by probing for the presence or absence of these structures. Stainless steel snake probes are commercially available and highly recommended. The probe should be lubricated with a commercial lubricating jelly to facilitate passage. The probe should be inserted caudolaterally (Figure 7-11), as far as it can be directed. Once the probe cannot be inserted any further, the handler’s thumb should be used to mark the position of the probe at the level of the vent. The probe should then be removed from the vent and placed on the external surface of the tail. If the probe travels farther than five scales, than the snake is (generally) a male. If the probe does not travel farther than five scales, then the snake is a female. Male snakes generally have longer and wider tails than do females. I will always probe the second side if the first probing suggests a female. Juvenile snakes can be sexed by gently everting a hemipenis. This technique is usually reserved for hatchlings. Holding the tail of a snake up to a bright light can also reveal the presence or absence of hemipenes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree