Chapter 14
Cerebrospinal Fluid and Central Nervous System Cytology
Cerebrospinal Fluid
Cerebrospinal fluid (CSF) is present within the ventricular system of the brain, the central canal of the spinal cord, and the subarachnoid space (SAS) between the pia mater and arachnoid mater. CSF is a component of, and is continuous with, the interstitial fluid of the central nervous system (CNS). It is separated from the bloodstream and from the CNS parenchyma by an intricate barrier system comprising ependymal epithelium, choroid plexus epithelium, the leptomeninges, areas of modified leptomeninges, and the arachnoid villi (the reader is referred elsewhere for a thorough discussion of these barriers and their transport mechanisms).1,2 CSF has mechanical (protection) and metabolic (transport, excretion) functions. Sampling of the CSF is an important part of the minimum database for patients with neurologic signs and may be useful in monitoring response to therapy in CNS inflammatory disease. When performed correctly, acquisition is a rapid, inexpensive, and technically simple method of sampling the local environment of the CNS extracellular space for evidence of inflammatory, neoplastic, traumatic, or degenerative disease. It is not without risk, however, and should be performed judiciously, that is, when clinical indication exists and no contraindications are present. This chapter will review the biology of CSF, methods for collection, and causes for abnormalities in parameters such as protein concentration and nucleated cell count.
Limitations of Cerebrospinal Fluid Analysis
Analysis of CSF is an important adjunctive diagnostic tool in the workup of patients with CNS disease and must be interpreted within context of the patient’s history, clinical signs, clinicopathologic data, imaging studies, and other ancillary diagnostics. Rarely is CSF solely used to provide an etiologic diagnosis (exceptions include cytologic visualization of infectious agents or overtly neoplastic cells), but analysis may significantly narrow the field of pathophysiologic differentials, guiding further diagnostic and therapeutic options. CSF analysis is most sensitive in detecting inflammatory disease.3 Positive findings in CSF tend to be more diagnostically helpful compared with negative findings but are often nonspecific, as many different diseases may cause a common CSF pathology (e.g., neutrophilic pleocytosis).4 Occasionally, the magnitude of change within the CSF may be as instructive as the character of the change, for example, marked increase in protein concentration raising diagnostic concern for feline infectious peritonitis or marked neutrophilic pleocytosis raising diagnostic concern for steroid-responsive meningitis-arteritis in a young dog in pain.4 More frequently, however, specific disease etiologies will present with CSF changes of variable character and magnitude. CSF that falls within laboratory reference intervals should never be used to rule out a differential diagnosis, as negative findings may represent early or mild disease, disease suppressed or masked by therapeutic intervention, or a disease process that does not present within the particular area of the extracellular space being sampled. CSF analysis may or may not correlate with imaging studies; a retrospective study of 92 cats receiving magnetic resonance imaging (MRI) for spinal signs showed that abnormal CSF was not a predictor for abnormal MRI.5 In another study, approximately 25% of dogs with intracranial signs and inflammatory CSF had normal brain MRI.6 As with other clinicopathologic data, a single CSF sample is a static representation of dynamic physiology; therefore, repeat CSF analysis may be useful in monitoring emerging disease, response to therapy, or both and may help guide therapeutic options during long-term treatment. An example is the treatment of cryptococcosis, when antimicrobial therapy is recommended at least until the antigen latex agglutination titers of serum, CSF, or both fall to zero.7
Formation and Movement of Cerebrospinal Fluid
The conventionally accepted theory of CSF secretion and transport is based on the concept of active transport of ions within ventricular ependymal cells and choroid plexi, subsequent passive flow of fluid, and circulation and drainage of CSF into dural venous sinuses. These ideas have recently come under scrutiny as potentially simplistic and inconsistent with the last 100 years of experimental evidence.8 Analysis of past experiments, coupled with new data, supports a “global production” hypothesis—that instead of exclusive formation within the ventricles, CSF is continually created and reabsorbed diffusely by cerebral capillaries that have slight variances in hydrostatic and osmotic pressure. Canine studies have documented CSF production within the ventricular system and the SAS.2
Contraindications to Acquisition of Cerebrospinal Fluid
CSF should not be collected from patients with unacceptable anesthetic risk or with suspected coagulopathy, severe cervical trauma, or increased intracranial pressure secondary to edema, hemorrhage, hydrocephalus, or a large neoplasm.9 CSF collection in the presence of elevated intracranial pressure may cause brain herniation and death secondary to compression of respiratory centers.10 Signs of increased intracranial pressure may include: stupor, coma, bradycardia, systemic hypertension, cranial nerve deficits, rigid paresis, or all of these.11,12 Mannitol and hypertonic saline are the first-line medical therapies for elevated intracranial pressure. Head elevation, modest hyperventilation, administration of drugs to slow brain metabolism, and craniectomy with durectomy are sometimes used in cases refractory to traditional treatments. Advanced imaging before CSF collection, especially in patients presenting with intracranial neurologic signs, may be useful in identifying contraindications. Imaging, in particular MRI, is exquisitely helpful in providing structural data which may be correlated to CSF results.
Collection Techniques
Cerebromedullary Cistern versus Lumbar Cistern
A study of 158 dogs with focal, noninflammatory disease showed that in cases of spinal lesions, CSF was more likely to be abnormal if collected from the lumbar cistern, that is, caudal to the lesion.13 This observation may be explained by presupposing cranial to caudal flow of CSF, but the traditionally held theory of CSF flow has recently been contested.8 In canines, CSF collected from the cerebromedullary cistern generally has lower microprotein concentrations compared with samples collected from the lumbar cistern.14 Blood contamination may be more pronounced in lumbar collection, as the desired subarachnoid space is more difficult to enter and yields a smaller volume of fluid that tends to flow more slowly.10,11 Moreover, hemodilution may contribute to increased measured protein concentration.14
Rare instances of CSF contamination with hematopoietic precursors have only been reported from lumbar sites.15 A low, but potentially catastrophic, risk of puncturing the cervical spinal cord or caudal brainstem exists during cerebromedullary collection. Because the spinal cord length is variable, spinal cord puncture is a possibility during lumbar collection, but it is associated with less severe adverse effects compared with injury following cisternal puncture. In a case series of 4 accidental cisternal parenchymal punctures (documented by MRI), 3 out of the 4 patients suffered neurologic decompensation and subsequently had to be euthanized.16
Cerebrospinal Fluid Acquisition
The anesthetized patient is placed in lateral recumbency (it is generally easier for a right-handed clinician to have the patient in right-lateral recumbency, and vice versa), with the neck and back flush to the edge of a sturdy table. For collection from the cerebromedullary cistern, the neck is flexed such that the dorsum of the muzzle is 90 degrees to the long axis of the body (stabilizing the endotracheal tube, if needed, to prevent kinking and deflating the cuff, if necessary, to prevent tracheal trauma), and the snout is propped up slightly, if necessary, to keep it parallel with the table and not angulated from the sagittal plane.11 A wide area (3 to 5 cm) around the atlanto-occipital joint (beyond atlas wings and axis spinous process and to the external occipital protuberance) is shaved and aseptically prepared, and landmarks are palpated with a gloved, nondominant hand.10 The needle is inserted at the intersection of two imaginary perpendicular lines that run (1) along the dorsal midline (dividing the patient sagitally) from the occipital protuberance to the cranial spinous process of the axis (C2) and (2) across the craniolateral aspects of the wings of the atlas (C1) (dividing the patient craniocaudally).
For lumbar collection, the pelvic limbs are brought forward into full flexion, and the needle is inserted cranial and parallel to the dorsal spinous process of L6 for dogs and L7 for cats advancing the needle until the ventral aspect of the vertebral canal is encountered; the needle is then retracted slightly and CSF is collected from the ventral SAS.11,17 The pelvic limbs may kick or twitch slightly during collection because of irritation of the cauda equina or spinal cord parenchyma.
For either location, once landmarks are palpated, the needle is held stably with the dominant hand and very slowly advanced, stylet in place. The heel of the dominant hand may be supported against the table. For cisternal collection, it is important to advance the needle toward the point of the nose without angulation. The stylet is removed with the nondominant hand every 2 to 3 mm to check for fluid within the needle hub, waiting a few seconds. It is common to feel a decrease in resistance to forward needle movement once the thecal space is entered. If bone is hit or frank hemorrhage is observed from the needle, it should be withdrawn slowly and collection reattempted.10 If clear or slightly blood-tinged fluid is observed, advancement of the needle ceases and open tubes are placed directly under the needle hub to collect freely falling drops.
No significant objective data exist regarding the maximal amount of CSF that may be collected in dogs. Several authors claim that it is safe to collect 0.2 milliliters (mL) of CSF per kilogram of body weight (1 mL/5 kg); in other species much higher volumes of CSF per body weight are acquired standardly.18 In general, 0.5 to 1 mL of CSF is adequate for routine diagnostic tests, including cell counts, protein concentration, and cytologic analysis. Larger volumes are necessary for additional diagnostics (cultures, titers, polymerase chain reaction [PCR], protein electrophoresis, etc.).
CSF is collected passively and should not be aspirated. Free drops are allowed to fall from the hub of the needle into an open tube placed just below. Two sets of tubes should be readied and ideally handled by an assistant. An ethylenediaminetetraacetic acid (EDTA)–treated (purple top) tube is used for cell counts and PCR testing for organisms, and plain (red top) tubes are used for protein concentration, culture, or immunologic assays.11 Some sources indicate that plain tubes are recommended, as EDTA could increase protein concentration. If CSF analysis will occur rapidly (within 1 hour), collection into a plain tube is adequate, whereas preservation of cells may be improved with collection into EDTA if analysis will be delayed. If low volume is present, priority is given to the EDTA tube. If CSF appears red, then iatrogenic hemorrhage (puncture of a dural vessel) or actual CNS hemorrhage has occurred. In this instance, the first few drops are allowed to collect into the first set of tubes, and the second set of tubes are reserved for the latter portion of the sample, as iatrogenic hemorrhage tends to clear over time. If the hemorrhage does clear, a decision may be made about discarding the first set of tubes or keeping them for ancillary testing not affected by the hemorrhage. After collection, the needle is withdrawn without the stylet, and the CSF within the needle is allowed to drip into one of the tubes or is placed in an additional plain tube and saved for culture.
Cerebrospinal Fluid Processing and Analysis
As with other clincopathologic and cytologic samples, evaluation of a fresh specimen is preferred to minimize cellular degradation, to which CSF is particularly vulnerable because of its relatively low protein concentration. Sample degradation will affect cell differential count to a greater extent than the total nucleated cell count or the protein concentration.19 A study of 30 canine CSF samples with pleocytosis concluded that delay of analysis up to 8 hours was unlikely to alter interpretation, especially in samples with protein concentrations above 50 milligrams per deciliter (mg/dL).19 Preservative should be added to low protein samples unless analysis is to be completed within 60 minutes (see next section), and a dilutional effect must then be factored into cell counts.19 Samples to be shipped to a reference laboratory overnight should be kept at refrigeration temperature and shipped with ice packs for analysis within 48 hours.10,17 The reference laboratory should be prenotified to ensure prompt analysis.
If analysis is to be delayed by more than 1 hour and the CSF sample has a protein concentration less than 50 mg/dL, one of the following may be added as a protein source to maintain cellular integrity: (1) hetastarch (add 1:1 volume), (2) fetal calf serum (3.7 g/dL protein; add 20% by volume), or (3) autologous plasma or serum (fresh or frozen; 11% by volume = one drop from 25-gauge needle (approximately 0.03 mL) mixed into 0.25 mL CSF).11,20,21 One study demonstrated better preservation of mononuclear cells in canine samples when fetal calf serum was used instead of hetastarch.19 All samples should be refrigerated at 4°C to minimize cellular degradation.
Cell Counts
A hemocytometer may be employed in practice to count nucleated cells and erythrocytes. Both sides of the cover-slipped hemocytometer are loaded with unstained CSF, which is then placed in a humidified container for 10 to 15 minutes to allow cells to settle on the glass. Because the fluid is unstained, the microscope condenser is lowered to improve contrast. Erythrocytes and nucleated cells are differentiated by size, refraction, granularity, and smoothness of plasma membrane.22 Some laboratories stain CSF samples with new methylene blue (NMB), as leukocytes will take up stain while erythrocytes remain unstained, making differentiation of leukocytes and erythrocytes easier (Figure 14-1).23 A small volume of CSF is drawn into a capillary tube coated with NMB or a tube which has a small volume of NMB followed by an air pocket.23 The tube containing NMB and CSF is gently rocked back and forth, allowing the cells to take up some stain without diluting the CSF with a volume of NMB.23 The hemocytometer is then loaded, and each population is counted and totals are calculated, as follows: Neubauer chamber: (1) Count both areas of large nine squares, and find the average of the number of leukocytes and erythrocytes; (2) multiply the average by 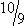 to get the cells per microliter (cells/µL).11
to get the cells per microliter (cells/µL).11
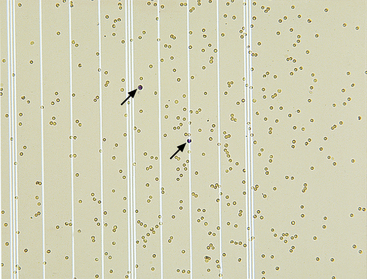
Figure 14-1 Numerous erythrocytes and two leukocytes present on a hemacytometer.
The two nuclei of the two leukocytes in the center of the field stain dark purple (arrows). (New methylene blue stain. Original magnification 50×.)
The ADVIA 120 (Siemens Medical Solution, Fernwald, Germany) hematology instrument has been validated for analyzing canine CSF samples and shows excellent correlation with manual methods used in dogs with increased total cell counts (pleocytosis), but the instrument may overestimate the cell count in samples without pleocytoses and has not been validated for the identification of eosinophils.24 The automated differential count is also more accurate at higher cell numbers and, thus, should be compared with a traditional manual differential. The ADVIA 2120 hematology analyzer displayed satisfactory agreement with the standard hemocytometer method.25 Validation experiments using 67 canine samples showed a sensitivity of 100% and specificity of 89% for accurately identifying samples with pleocytosis when manual counting was considered the gold standard (>5 cells/µL).25 The instrument tended to be less accurate at lower (within reference interval) nucleated cell counts.25 Erythrocytes may be a source of interference, as a red blood cell (RBC) count of 250 cells/µL was shown to elevate the nucleated cell count. With regard to differential cell count, the instrument performed better in the presence of pleocytosis, whereas monocytes were overcounted at lower nucleated cell counts.25 Automated cell counts should, thus, not replace a manual differential but may be used as another level of quality control. Automated instruments cannot recognize altered cell types such as atypical neoplastic cells.
Measurement of Microprotein Concentration
Measurement of CSF specific gravity is not considered to be helpful because of low sensitivity for detecting abnormalities.12 CSF microprotein may be semiquantitatively measured by using urine dipsticks that detect albumin. This assay has a lower detection limit of 100 mg/dL; therefore, it has low sensitivity for mild to moderate CSF protein concentration elevations (30 mg/dL to 100 mg/dL). False-positive or false-negative reactions may occur if the dipstick reads at trace or 1+, but this method is useful if other techniques are not available.11 Reference laboratories apply a similar, but more sensitive, methodology to measurement of CSF microprotein as serum protein, using the trichloroacetic acid method, the Ponceau S red dye binding method, or the Coomassie brilliant blue method.22
Screening tests for CSF globulins include the Nonne-Apelt (also called Ross-Jones) test and Pandy’s reaction. In the Nonne-Apelt test, CSF is added to the top of 1 mL saturated ammonium sulfate and left standing for 3 minutes at room temperature. The presence of a white-gray ring at the interface is a positive reaction. In Pandy’s reaction, a few drops of CSF are added to 1 mL of 10% carbolic acid solution and the resulting turbidity is graded 0 to 4. Any Pandy score above zero is considered elevated. Globulin concentration below 50 mg/dL will be undetectable with either test.11,22
Protein electrophoresis and immunoelectrophoresis may be performed on CSF as well as serum for maximum fractionation.26 The utility of protein electrophoresis or immunoelectrophoresis of CSF lies in discriminating altered blood–brain barrier (BBB) permeability from increased localized production of immunoglobulin, which may be suggestive of (but not specific for) a disease entity for which an electrophoretic pattern has been established.
Cytologic Slide Preparation
Use of an in-house sedimentation chamber (Sörnäs procedure) may be very useful and preserves cell-free fluid for ancillary testing.11 This technique will recover approximately 60% of total cells, which is sufficient for analysis.17 A syringe barrel (with the tip and needle aseptically removed with a scalpel blade) is turned upside down and the smooth, top side is placed in warm petroleum jelly and then onto a clean slide. Once a seal has formed, fresh CSF (at least 0.5 mL) is placed in the syringe and allowed to sit for 30 minutes.17,22 Then, the supernatant is aspirated carefully with a pipette so as not to disturb the bottom layer contacting the slide. The syringe barrel is removed, and any excess CSF is carefully absorbed with a small piece of filter paper or paper towel. The slide is completely and rapidly air-dried without heat (inadequate drying results in cellular distortion), excess petroleum jelly removed with a scalpel blade, and the slide is stained with routine Romanowsky stains (e.g., Diff-Quik).
If CSF is sent to a reference laboratory, a cytologic slide will likely be prepared using cytocentrifugation (500 to 1000 revolutions per minute [rev/min] for 5 to 10 minutes, either onto a slide coated with albumin or with the addition of 0.05 mL of 30% albumin for improved cell capture) for maximal concentration of nucleated cells onto one slide.17 Cytocentrifuged cytologies have excellent cellular detail, but the preparation may enlarge cells slightly and create an artifactual foamy or vacuolated appearance.17 Slides are air-dried and stained with conventional Romanowsky stains. Multiple cytospin preparations may be made to yield 200 intact nucleated cells for classification.
Additional Cerebrospinal Fluid Testing
Culture
As it is rare for etiologic agents to localize only within the CNS, all cases of suspected infection may be aided diagnostically by fine-needle aspiration (FNA) cytology, biopsy with histopathology, culture of nonneural lesions, or all of these.21 Bacterial culture and sensitivity testing of CSF is recommended for most cases of neutrophilic pleocytosis given appropriate clinical index of suspicion for a septic lesion. Even when organisms are visualized on CSF cytology, speciation and sensitivity testing may guide prognostic and treatment decisions. Alternatively, bacterial or fungal culture may be negative regardless of cytologic observation of organisms.11,21 It must be remembered that bacterial CNS infection is highly uncommon in dogs and cats compared with other domestic animal species.27
Titers and Polymerase Chain Reaction Testing for Infectious Agents
Advanced techniques for neurologic disease diagnosis are expanding rapidly. Enzyme-linked immunosorbent assay (ELISA)–based assays for antibody detection and PCR-based assays for nucleic acid detection of several medically important microbes have been developed for use on CSF and may be instructive in the diagnosis of viral, rickettsial, protozoal, or fungal diseases.21 A large canine study that included a subset of 16 dogs with neoplastic or inflammatory disease showed that CSF titer provided diagnosis in 25% of cases.3 Antibody assays should be interpreted cautiously, as the presence of antibody may indicate prior exposure rather than active infection. Moreover, compromise to the BBB in states of inflammation may translate to the presence of antibodies within the CSF without local production. Occasionally cross-reactive antibodies may be present that do not represent presence of the disease agent under assessment. Similarly, specimens for PCR should be submitted to a laboratory with strict quality control to minimize false-negative and false-positive results. Poor collection technique may result in false-positive results, especially for bacterial species that are ubiquitous in the environment.28 As with other aspects of CSF analysis, a negative PCR result does not definitively rule out the presence of a pathogen because of the sampling limitation of a small portion of the extracellular space.21
Enzymes, Neurotransmitters, and Other Molecules
CSF contains glucose, electrolytes, neurotransmitters, and enzymes, but these substances are not measured routinely, although this measurement represents a rapidly expanding area of research in the effort to give clinicians better tools for diagnosing patients as well as determining prognoses. CSF enzymes originate from the bloodstream, the CNS, or cells within CSF.11 One study of 34 cats with noninflammatory CNS disease showed that measurement of CSF activities of lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and creatine kinase (CK) were not diagnostically sensitive but may be useful in detection of acute injury.29 Multiple studies have correlated elevations in CSF CK activity with poor prognosis in dogs with neurologic disease or spinal cord injury.30,31 Immunoassays for vascular endothelial growth factor (VEGF) and S-100 calcium-binding protein have shown elevations of both molecules in the CSF of experimentally induced hypothyroid dogs, suggesting endothelial and glial contribution to increased BBB permeability in this population.32 Myelin basic protein (MBP) has been found to be elevated in lumbar CSF in dogs with degenerative myelopathy, supporting the conclusion that it is a demyelinating lesion.33 Myelin basic protein concentration is elevated in the CSF of dogs affected by intervertebral disc herniation (IVDH) and has been found to be an independent predictor of poor prognosis.34 Beta-2-microglobulin, a major histocompatibility complex I (MHC-I)–associated molecule, has been assayed by using ELISA and found to be elevated in the CSF of dogs with IVDH and inflammatory disease and also positively correlated with normal total nucleated cell count (TNCC).35 The amino acids tryptophan and glutamine have been found to be elevated in the CSF of dogs with portosystemic shunts because of abnormal ammonia metabolism.36 One study found increased oxytocin in the CSF of dogs with spinal cord compression, where it is believed to have an analgesic effect.37 Gamma-aminobutyric acid (GABA) and glutamate neurotransmitter concentrations have been measured in dogs with epilepsy.38
Normal Cerebrospinal Fluid Parameters
Gross Examination
Normal CSF is clear and colorless, with few cellular elements and a protein concentration approximately 200 to 300 times less than that of plasma or serum. Red or yellowish coloration indicates prior lesional hemorrhage or iatrogenic hemorrhage during collection. In the latter case, a pellet of red cells will be present after centrifugation. True xanthochromia (yellowish color of hemoglobin breakdown products) that does not clear on centrifugation, cytologic evidence of erythrophagia, or both, indicate prior hemorrhage into the subarachnoid space.21 Increased bilirubin leakage into the SAS or high concentrations of CSF protein (>100 to 150 mg/dL) may cause xanthrochromia.22 Increased turbidity of the sample may be caused by increased number of cells present (>400 RBCs/µL or >200 nucleated cells/µL) but is usually not affected by mild changes.11,12
Cell Counts
TNCC is fewer than 5 cells/µL in the dog and fewer than 8 cells/µL in the cat, and elevation above this range is termed pleocytosis.11 Grading of pleocytosis is somewhat subjective: In one reference, “mild” was defined as 6 to 50 cells/µL; “moderate” as 51 to 1000 cells/µL; and “marked” as more than 1000 cells/µL.4
Microprotein Concentration
Depending on laboratory-specific reference intervals, normal protein concentration is usually less than 25 to 30 mg/dL for cisternal CSF and less than 45 mg/dL for lumbar CSF.11,21 Approximately 80% to 95% of CSF protein is albumin, and 5% to 12% of CSF total protein comprises gammaglobulins.2 Eighty percent of CSF protein is transferred from plasma, with the remainder produced within the CNS. The latter population includes molecules also produced by other organs and proteins unique to the CSF that may potentially be used as markers of CNS tissue damage. Experimental evidence and earlier literature support a gradient of increasing protein concentration from cranial to caudal within the subarachnoid space, which has been attributed to slower flow and greater blood–CSF permeability caudally.13
Normal Cytology
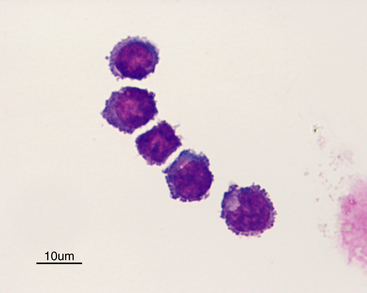
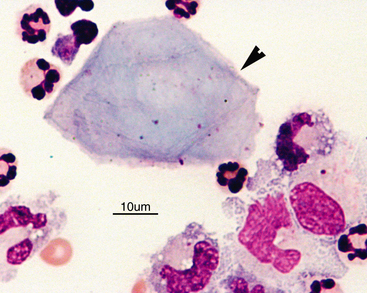
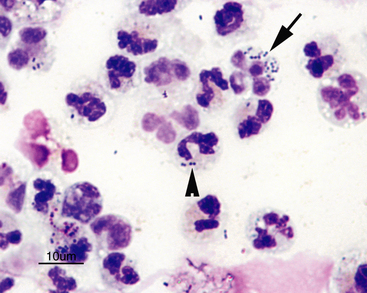
Normal CSF is acellular or contains small numbers of small lymphocytes (Figure 14-2 and Figure 14-3) and large mononuclear cells (macrophages, ependymal lining cells, meningothelial lining cells, choroid plexus cells) (Figure 14-4 and Figure 14-5). Large mononuclear cells may be vacuolated and contain phagocytized material (Figure 14-6). A low frequency of nondegenerate neutrophils (< 25%), which are usually indicative of blood contamination during collection, may be present.39 A study of 359 samples of canine CSF found a 7.5% incidence of meningeal, choroid plexus, ependymal, endothelial cells, or all of these. No correlation existed between the presence of these cells and the presence of pleocytosis, elevated protein concentration, or the primary disease etiology.40 Thus, it is postulated that the presence of these cells is an artifact of collection and should not be overinterpreted. The authors recommended the term “surface epithelial cells” for the combined grouping (which cannot be distinguished cytologically), although not all of these cells (meningeal, endothelial) are of epithelial origin.40 Occasionally, anucleate superficial squamous epithelial cells may be seen; these may be caused by contamination from the skin (Figure 14-7).
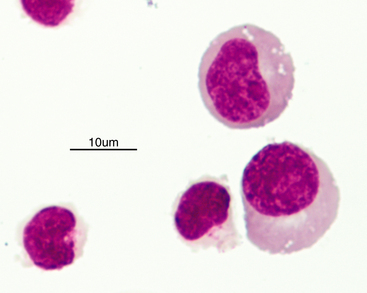
Figure 14-3 Small lymphocytes in a cerebrospinal fluid sample.
The two cells to the right have slightly increased amounts of cytoplasm. (Wright-Giemsa stain.)
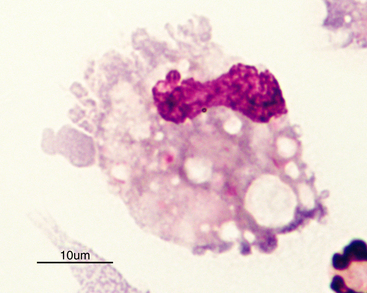
Figure 14-4 Large mononuclear cell with cytoplasmic vacuolation in a cerebrospinal fluid sample. (Wright-Giemsa stain.)
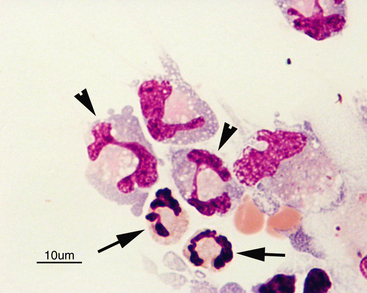
Figure 14-5 Numerous large foamy mononuclear cells and two neutrophils (arrows) in a sample of cerebrospinal fluid.
Large mononuclear cells may have nuclei that are similar in shape to band cells or neutrophils, only larger (arrowheads). (Wright-Giemsa stain.)
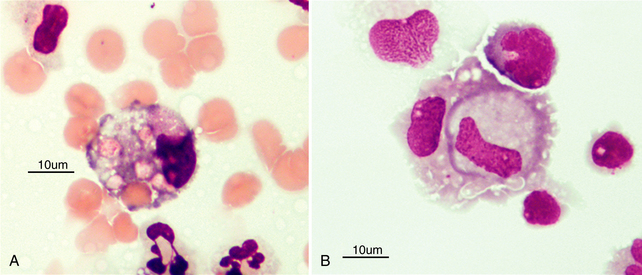
Figure 14-6 Large mononuclear cells in cerebrospinal fluid showing evidence of (A) erythrophagia and (B) leukocytophagia. (Wright-Giemsa stain.)
Other Parameters
Occasionally, small amounts of granular, foamy extracellular material are present and are consistent with myelin or myelin-like material, which will stain positively with Luxol fast blue. This material may consist of myelin fragments, which are generated from demyelination, or may consist of myelin figures (a nonspecific term for layered phospholipids exfoliated from damaged cells).41 The two cannot be distinguished with light microscopy. The significance of this material remains unclear, as it may be observed in samples from patients with no discernible cause. A study of 98 canine cerebromedullary and lumbar CSF samples showed 20% incidence of myelin-like material, with a higher percentage in samples from the lumbar cistern or from small dogs (<10 kg).42 The presence of the material was not correlated with case outcome.42 Similarly, in a study of 61 Cavalier King Charles Spaniels with Chiari-like malformations, myelin-like material was observed in 57% of lumbar CSF collections and 12% of cerebromedullary collections.43 Thus, myelin-like material may be a procedural artifact or may be consistent with a demyelinating (e.g., canine distemper virus, degenerative myelopathy) or potentially necrotizing disorder (e.g., IVDH, other spinal trauma, or a necrotic neoplasm).41,42
Interpretation of Abnormal Cerebrospinal Fluid
Blood Contamination and Hemorrhage
Normal CSF should not contain erythrocytes, but hemodilution is a common occurrence. Varying reports on the effect of blood contamination on TNCC, leukocyte differential, and protein concentration have been published.44–47 Deciding whether increased TNCC or protein concentration is the result of hemodilution alone or a significant change concurrent with hemodilution necessarily remains, to an extent, a subjective assessment and must be critically evaluated in light of the magnitude of CSF findings along with the other pertinent facts of the case. One study in dogs determined that up to 13,200 erythrocytes/µL should not significantly affect TNCC or microprotein concentration, but feline CSF may be more sensitive to blood-induced changes.44,45 Correction formulas for CSF parameters in the face of hemodilution (e.g., adding 1 nucleated cell/μL per 100 or 500 RBCs/µL) are unreliable.46,47 In a recent study of 106 canine CSF samples without pleocytosis (TNCC <5/µL) but containing at least 500 RBC/µL, the mean percentage of neutrophils (45.2% versus 5.7%), percentage of samples with eosinophils present (36.8% versus 6.8%), and mean protein concentration (40 mg/dL versus 26 mg/dL) were found to be significantly increased in the samples with blood contamination compared with controls.48 Significant RBC contamination warrants repeat sampling, if possible. Marked hemorrhage or evidence of prior hemorrhage (erythrophagocytosis, xanthochromia, hemosiderin-laden macrophages) may be useful in the diagnosis of CNS trauma, which may be accompanied by neutrophilic to mixed cell pleocytosis and mild increase in protein concentration.4
Elevated Microprotein Concentration
Elevated protein concentration in CSF (>30 mg/dL) may occur with or without pleocytosis, and in the absence of pleocytosis, is termed albuminocytologic dissociation (ACD). High protein concentration may be the result of leakage of plasma or cellular proteins across the BBB, localized production of immunoglobulin, localized tissue damage or necrosis, decreased clearance of protein into the venous sinuses, obstruction of CSF circulation, or all of the above. As such, it is a nonspecific change that indicates CNS damage or hyperproteinemic disease and is consistent with disease of any etiology (e.g., trauma, metabolic, infectious, inflammatory, degenerative, or neoplastic). Caution should be exercised when diagnosing ACD if the sample is hemodiluted (> 500 RBC/µL).48 As is true for pleocytosis, inflammation of the meninges and superficial regions of parenchyma will result in greater CSF protein elevations than for lesions that are more remote from the SAS.
Alterations of Leukocyte Percentages without a Pleocytosis
Occasionally, an abnormal leukocyte differential (shifted from mononuclear predominance to neutrophil predominance) without pleocytosis occurs. This may only be detected if cytologic analysis (after sedimentation or cytocentrifugation of CSF) is performed. Increased percentages of neutrophils may occur in early or mild inflammatory disease, noninflammatory CNS disease, disease that is remote from the SAS or sampling site, or in cases of hemodilution. An increased proportion of neutrophils is present when neutrophils comprise greater than 25% of all nucleated cells, and increased percentage of eosinophils occurs when eosinophils comprise greater than 1% of the differential.11
Increased Neutrophil Percentage
When present (with or without increased TNCC), neutrophils should be evaluated for toxic change, degenerative change, and intracellular organisms or other inclusions (Figure 14-8). Increased percentage of neutrophils without pleocytosis has been associated with healthy dogs, blood contamination, degenerative disc disease, neoplasia, cerebrovascular accident, fracture, and fibrocartilagenous embolism (FCE).11,43 A study of 61 Cavalier King Charles Spaniels with Chiari-like malformation documented that those with syringomyelia were more likely to have an increased percentage of neutrophils, but it was not reported whether this subpopulation also had a concurrent pleocytosis.43 In another study, cats with CNS neoplasia had increased percentage of neutrophils or lymphocytes without a pleocytosis.29 Although not a classic pattern, infectious or inflammatory disease should not be ruled out if increased neutrophils are visualized without pleocytosis.
Increased Eosinophil Percentage
Increased percentage of eosinophils has been reported in parasitic and protozoal diseases such as Neospora caninum infection.39 One cat with eosinophilic meningoencephalitis (EME) of unknown etiology had an increased percentage of eosinophils and lymphocytes without pleocytosis.49
Increased Nucleated Cell Counts (Pleocytoses)
The specific diseases mentioned in the next section on various categories of pleocytosis are a survey of the current literature and meant to be a helpful starting point in the generation of particular differential diagnoses. Thus, disease entities are listed in the section under which they are most commonly present, but it is important to note that for all disease entities, variability in the nature and the magnitude of pleocytosis may emerge in a particular patient at a particular point in time. Wherever possible, other categories of pleocytosis that have been reported for a disease have been mentioned. Generally, pleocytoses are defined by the cell type that comprises 70% or more of the nucleated cell population. If all cell types are 50% or less, the pleocytosis is classified as a mixed cell pleocytosis. And if, for example, lymphocytes are greater than 50% but less than 70%, some pathologists will classify the pleocytosis as mixed cell, lymphocyte predominant. A pleocytosis will be classified as eosinophilic if eosinophils compose at least 10% to 20% of the nucleated cell population.12
Neutrophilic Pleocytosis
Infectious Conditions
Bacterial Meningoencephalomyelitis
Bacterial infections of the CNS are unusual and represent a small portion of neutrophilic pleocytoses. Typically, this pleocytosis is severe (could be over 1000 cells/µL), neutrophilic, and accompanied by significantly elevated protein concentration, but the cell population may change to mononuclear during the course of treatment.11,21,50 Rare instances of brain abscessation secondary to sepsis (which may be a sequela of iatrogenic immunosuppression) may result in marked neutrophilic pleocytosis, markedly elevated protein concentration, visualization of bacterial organisms (Figure 14-8), and abnormal MRI findings.51 Staphylococcus intermedius was cultured from the CSF of a dog presenting with a retrobulbar abscess and neurologic signs.52 The CSF showed a moderate neutrophilic pleocytosis (75 cells/µL) and borderline elevation in protein concentration (30 mg/dL).52 Pasteurella multocida meningoencephalomyelitis in a kitten was characterized by marked neutrophilic pleocytosis (981 cells/µL) with mild protein elevation (31 mg/dL) and rare extracellular and intracellular bacterial rods.53 Bacterial culture and sensitivity testing are recommended but may have false-negative results if organisms are not circulating in the extracellular space or if prior antibiotic therapy had been given. Serology and CSF-PCR (using organism-specific or universal bacterial [UB] PCR), are recommended.28,53
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree