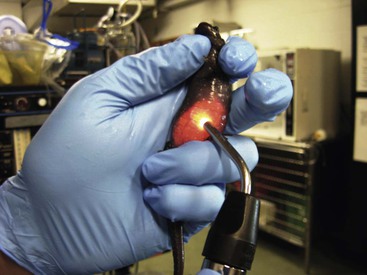
Eric J. Baitchman, Timothy A. Herman The order Caudata comprises 10 families of salamanders, the tailed amphibians (Table 2-1).4 The earliest fossil record for this group dates back to the Jurassic period, over 150 million years ago. Their present distribution is primarily Holarctic, limited to the northern hemisphere regions of North and Central Americas, Europe, Asia, and northern Africa, with relatively few species occurring below the equator in South America. The largest family within the order is, by far, the Plethodontidae, a diverse group of lungless salamanders, containing nearly 70% of all species of Caudata. TABLE 2-1 Families of Caudata As the name implies, the unifying anatomic feature of this order is the presence of a tail. The plethodontid salamanders exhibit tail autotomy as a defensive mechanism, and many have a visible constriction at the cleavage site near the base of the tail.55 Generally, Caudata species have four limbs, except for the Sirenidae, which have small forelimbs and no hindlimbs. The Amphiumidae have four small vestigial limbs with only one, two, or three digits per limb, varying by species. The larvae of salamanders and their relatives are distinguished from anuran tadpoles by the presence of external gills. As larvae undergo metamorphosis, the gills regress. Some fully aquatic species such as the axolotl (Ambystoma mexicanum), the Sirenidae, and the Proteidae, are neotenic, or pedomorphic, retaining the juvenile gills through adulthood. Lungs are reduced in the torrent salamander family, Rhyacotritonidae, and in the Central Asian salamander, Ranodon sibiricus, and lungs are completely absent in the Plethodontidae and in the clawed salamanders, Onychodactylus spp. All other members of Caudata, including fully aquatic and neotenic species, do possess lungs.21 Sexual dimorphism varies greatly among species, and visible differences between sexes may be subtle. Females may be larger and have wider coelomic cavities compared with males in some species, whereas males may be drastically larger in others with competitive mating systems. Many male European newts (i.e., Triturus spp., Ommatotriton spp.) display ornate dorsal crests during the breeding season. A variety of male secondary sexual characteristics have evolved in the Plethodontidae, including mental glands, tail glands, cirri, and hypertrophied jaw muscles, which may become exaggerated seasonally during breeding. Some newts develop darkly colored keratinized nuptial pads, or excrescences, along the forelimbs or hindlimbs to aid in gripping females. Some male salamanders and newts also develop swollen vents from enlargement of seasonally responsive cloacal glands for the production of spermatophores.21 The hyobranchial apparatus is dramatically adapted to rapidly project the tongue and associated skeletal elements out of the mouth as a ballistic feeding mechanism in several genera of the Plethodontidae. In Hydromantes, tongue protraction is driven by dorsolaterally positioned subarcualis rectus muscles that extend from the floor of the mouth caudally past the forelimbs. The tongue is retracted by the rectus cervicis profundus muscles originating on the posterior pelvis and continuously running along the ventral abdomen to the tongue pad and are coiled near the heart between feeding events. Such essential feeding structures should be considered during any invasive procedure on these lungless salamanders. Mechanisms of respiratory exchange in Amphibia are remarkable for the taxa as a whole and may occur via four routes: branchial, buccopharyngeal, cutaneous, or pulmonary. The Caudata are unique in the extent to which different families have adapted to different primary routes. Branchial respiration is present in all amphibians as larvae, whereas only some neotenic salamander species retain this means of respiration as a primary route through adulthood. Cutaneous respiration is also employed by all amphibians to various degrees, although to a greater extent in caudates than in anurans. In anurans, cutaneous respiration occurs primarily as a means of carbon dioxide exchange, with the majority of oxygen exchange occurring in the lungs.21,31 Most caudates, by comparison, take up most of their oxygen through cutaneous respiration, even in species that possess lungs.58 Respiratory capillaries are concentrated in the skin in taxa that rely on the cutaneous route as the primary site for gas exchange, as in the lungless Plethodontidae and aquatic Cryptobrachidae. The cryptobranchids also use modified skinfolds to increase surface area and vascularization to enhance respiratory exchange underwater.31 Most salamanders come from habitats with relatively stable thermal environments. These temperatures, by and large, are substantially lower than the preferred temperatures of many frogs, with normal activity and feeding in most caudates typically occurring between 10° C and 18° C. Depending on the species in question, surface activity and even reproduction may occur at temperatures near freezing. Air conditioning and water chillers are required to maintain desired temperatures in most facilities. Thermoregulation in most species is limited to selecting refugia of the appropriate temperature.21 Providing a thermal gradient within the enclosure is optimal, allowing the animal to choose its preferred body temperature. Humidity levels which limit water loss are a critical component of refugia selection by terrestrial salamanders and may be a more important criterion than temperature.58 In the case of all caudates, it is important to completely secure the enclosure to prevent escapes. Many species are highly adapted for climbing smooth or slippery surfaces and wedging into very small crevices. These traits translate to scaling glass or smooth plastic with ease and exploiting any gap around the lid, drain cover, or intake filter of a pump. Foam weather stripping, silicone sealant, or duct tape should be used to seal gaps around lids, and fiberglass screening to prevent salamanders from entering the plumbing. Large aquatic salamanders such as cryptobranchids, amphiumas, and sirens are powerful swimmers and may leap to knock off an unsecured tank lid. In the case of smaller species and juveniles, plastic shoeboxes, food storage containers, or plastic deli cups with tight fitting lids with minimal modification are sufficient to securely house small salamanders. Organic substrates should be thoroughly soaked in clean water and the enclosure established prior to introducing salamanders. As in a new aquarium, a cycle of bacterial and fungal colonization and sequential establishment occurs on these substrates when they become wet, with associated byproducts of nitrogen decomposition. Substrates should be regularly rinsed and allowed to “cycle” until any stagnant or foul smelling odors dissipate. The addition of springtails (order Collembola) to the substrate may also facilitate the decomposition of waste and serve as a supplemental food source for smaller salamanders. Most salamanders exhibit a nocturnal or crepuscular activity pattern and seek refuge under stones, logs, leaves, or woody debris or in burrows or rock crevices when not active. As such, suitable refugia should always be provided in a captive setting to mimic these environments. All cage furnishings, particularly large rocks, should be stably secured to prevent shifting, which could crush salamanders. Most salamanders are eager and enthusiastic feeders so long as the appropriate food items are provided. A salamander that frequently refuses food is likely suffering from compromised health or an inadequate environment. Unlike most frog species, many salamanders use olfactory cues in conjunction with movement to detect food. As a result, some species (typically aquatic taxa) will feed on nonliving foods, including frozen thawed insect larvae and even commercially available pelleted foods. Many aquatic salamanders and larvae use a lateral line system, similar to that of fish, to detect movement of prey underwater. By and large, live moving food items are more readily detected and eaten by all salamanders. Many caudates have occasionally been documented to eat other salamanders, and the risk of consumption of smaller taxa or conspecifics should be considered in husbandry. A broad diversity of invertebrates comprises the staple diet of most salamander species. Earthworms and nightcrawlers (Lumbricus terrestris and others) are an excellent food source for many terrestrial and aquatic taxa, although the “red wiggler” (Eisenia foetida) sold for bait and composting may be refused because of its production of yellow defensive secretions. Smaller worm species that may be used for larval and adult salamander food include California blackworms (Lumbriculus variegatus), tubifex worms (Tubifex spp.), whiteworms (Enchytraeus albidus), Grindal worms (Enchytraeus buchholzi), and microworms (Panagrellus spp.). Insects provide the staple diet of most terrestrial salamanders. In captivity, the most readily available and useful feeder insects include the domestic cricket (Acheta domestica), wax moth larvae (Galleria mellonella), house fly larvae (Musca domestica), fruit flies (Drosophila melanogaster and D. hydei), bean beetles (Callosobruchus maculatus), terrestrial isopods (woodlice), and springtails. Aquatic insect larvae form an important dietary component of many salamander larvae, although their availability is limited in captivity. Fly larvae such as bloodworms (family Chironomidae) and glassworms (family Chaoboridae) are occasionally available at pet stores, live or frozen as food for tropical fish. Mosquito larvae (family Culicidae) and other aquatic insect larvae may be locally collected for salamander food. The large aquatic taxa (cryptobranchids, Necturus, Amphiuma, and Siren) all include crustaceans, fish, and opportunistically other vertebrates in their diet. Fish, crayfish, and large earthworms may be regularly fed to the large aquatic species and occasionally rodents, although only as a component of a broader varied diet. Feeding exclusively fish may result in nutritional deficiencies, and frequent feedings of rodents will quickly result in obesity in many species. The sourcing of live aquatic foods from a “clean” source may be difficult, and this route of transmission should be investigated if disease and parasite issues persist in a captive collection. Crayfish, in particular, have been shown to be a vector for chytrid and may pose a significant infection risk to captive salamanders.36 The frequency of feeding salamanders should match the metabolic needs of the animals. Because of the cool temperatures at which most species are kept, weekly or twice weekly feedings are sufficient to maintain most of them. During seasonal cooling periods, these feedings may be reduced further or eliminated entirely, depending on the activity level of the salamanders. Besides obesity, most nutritional problems in salamanders may be avoided by providing a diverse diet. Occasional supplementation with a quality vitamin or mineral supplement designed for amphibians seems sufficient to maintain good dietary health. As with handling any amphibian, care should be taken to avoid damage to the delicate skin and mucous layer. Rinsed, nonpowdered, disposable gloves should be worn when handling the animals (Figure 2-1). Clean, moistened plastic sandwich bags or sealable bags may provide an effective and safe restraint for procedures such as radiography or to administer injections. Soft, nonabrasive aquarium nets are suitable for capture of aquatic species, taking care not to damage the delicate gills of neotenic species. Some species of salamander will attempt to spin or roll on their long axis when in hand, and along with the slippery mucous secretions from the skin, this behavior may make it very difficult to appropriately restrain the animal. Chemical restraint may be required to safely perform more than cursory examinations or treatments in some species. Restraining an animal by the tail should be avoided, as it may induce autotomy, especially if the animal begins a defensive rolling behavior. Indications for surgery are the same as with any other amphibian, and safe anesthesia practices are well established. Surgical cases have been reported for biopsies, endoscopies, gastric foreign bodies, mass removal, radiotelemetry implantation, and limb amputation in both clinical and research settings. Amputated limbs may regenerate in salamanders and newts, and amputation sites may be left open for normal regeneration, with topical care to prevent infection. Closure of the amputation site with a skin flap may cause abnormal regeneration or may prevent it completely.3 Intracoelomic surgery is best approached through a paramedian incision to avoid the ventral abdominal vein present on the midline. Skin closure is recommended with an everting pattern. Because of the aquatic environment of many species, use of nonabsorbable monofilament suture in an interrupted pattern is recommended to avoid premature dissolution of absorbable materials and potential dehiscence.2,59 Cyanoacrylate tissue adhesive is waterproof and may be used for primary closure or for additional protection.3,59 Anesthesia of amphibians has been summarized elsewhere.6 Special considerations for this order are particularly for the neotenic species, which may have shorter induction times compared with metamorphic adults, and for hellbenders, which require much lower induction doses compared with other amphibians.11 Tricaine methanesulfonate, benzocaine, and eugenol immersion baths, as well as injectable propofol, have all proven effective in salamander species.17,38,59 In the author’s experience, topical isoflurane baths have not provided a surgical plane of anesthesia in salamanders at doses described for anurans. Analgesia is provided as for other amphibians.6 Amphibians have served as models in analgesic research, revealing that the relative analgesic potency of mu, delta, and kappa opioid agonists are correlated with that seen in mammalian models.49 A specific study using Eastern red-spotted newts (Notophthalmus viridescens) corroborated other findings seen with opioid use in amphibians, that they require higher doses, have prolonged time to onset, and longer duration of action than in mammals. Given the delayed onset of action, early administration is recommended for opioid use, well ahead of anticipated need for analgesia.33 Table 2-2 lists anesthetic and analgesic agents that have been used in Caudata species. TABLE 2-2 Anesthetic and Analgesic Agents for Caudata Diagnostic sample collection and imaging may be performed similar to appropriate clinical investigations in any species. As for fish and other amphibians, water quality parameters should be measured as part of the basic workup, especially in aquatic species. The use of brief sedation should be considered when physical restraint is stressful to the animal or insufficient for completion of diagnostic procedures. Lighted magnification (such as with an otoscope head) is a useful aid in performing examinations. Transillumination is also useful for examination of the coelom, especially in less pigmented species; however, even in darkly pigmented species, it is usually possible to distinguish between fluid, air, or soft tissue coelomic distensions by this method. Transillumination is particularly useful as an aid in identifying the pathways of major blood vessels such as the ventral abdominal vein (see Figure 2-1). Blood collection is performed with small-gauge needles, 25-gauge to 27-gauge, and 1-milliliter (mL) syringes for most species. Heparinization of the syringe aids in preservation of small, slowly collected samples. The preferred site for most species is the ventral tail vein, approached perpendicularly on the ventral midline along the proximal third of the tail.2 The needle is advanced until contact with bone is made. A flash of blood indicates the appropriate location, and rotation of the syringe on its axis may better introduce the bevel of the needle into the vascular lumen for slow-flowing samples. One source suggests guidelines for selecting the tail vein in animals weighing greater than 10 grams (g) and using cardiac puncture in animals weighing between 4 and 10 g.50 The heart in salamander species is accessed just anterior to the thoracic girdle, almost in the caudal cervical region.50 The ventral abdominal vein may also be used in larger species. Anesthesia may be helpful for blood collection and is particularly recommended for cardiac puncture. Published reference ranges for selected species are listed in Tables 2-3 and 2-4.15,19,47,59 TABLE 2-3 Hematologic Parameters of Selected Caudata Species
Caudata (Urodela)
Tailed Amphibians
Biology
Taxonomy
Family Name
Common Name
Species
Ambystomatidae
Mole salamanders
34
Amphiumidae
Amphiumas
3
Cryptobranchidae
Giant salamanders
3
Dicamptodontidae
Pacific giant salamanders
4
Hynobiidae
Asian salamanders
59
Plethodontidae
Lungless salamanders
435
Proteidae
Mud puppies, olms, and water dogs
6
Rhyacotritonidae
Torrent salamanders
4
Salamandridae
True salamanders and newts
98
Sirenidae
Sirens
4
Unique Anatomy
Special Physiology
Special Housing Requirements
Feeding
Restraint and Handling
Surgery and Anesthesia
Agent
Dosage
Notes
Tricaine methanesulfonate (MS-222)
Larvae: 0.2 g/L
Adults: 1 g/L
Hellbenders: 0.25g/L
Solutions must be buffered to pH 7.0
Eugenol
450 mg/L
Surgical anesthesia in Ambystoma tigrinum
Benzocaine
Larvae: 0.05–0.1 g/L
Adults: 0.2–0.3 g/L
Paedomorphic adults induce faster than metamorphic adults
Propofol
35 mg/kg intracoelomic
Surgical anesthesia in Ambystoma tigrinum; prolonged induction
Buprenorphine
50 mg/kg, subcutaneously
>4 hr duration in Notophthalmus viridescens
Butorphanol
0.5 mg/L bath
Given as a continuous 72-hr bath in Notophthalmus viridescens
Diagnostics
Parameter
Ambystoma mexicanum (Metamorphosed)
Ambystoma mexicanum (Immature)
Plethodon cinereus
Notophthalmus viridescens
Cryptobranchus alleganiesis
Cynops pyrrhogaster
PCV (%)
30.00
27.7
—
—
31–47
40.0
Hemoglobin (g/dL)
7.6
7.5
—
—
10.7–8.32
—
MCV (fL)
—
8000–12,000
—
—
7425
—
Leukocytes (×103/µL)
2.5
2–3
—
—
2.6–6.4
1.8
Heterophils (%)
20–30
20–40
21.7
24.3
32–54
2.8
Lymphocytes (%)
40–60
30–50
65
63.5
28–58
3.0
Eosinophils (%)
2–10
0–10
3.6
6.2
0.5–19
4.0
Monocytes (%)
—
0.2
0.8
2.8
0–1.3
6.0
Basophils (%)
—
0–10
8.8
3.2
3–19.5
57.0 ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Caudata (Urodela)
Chapter 2