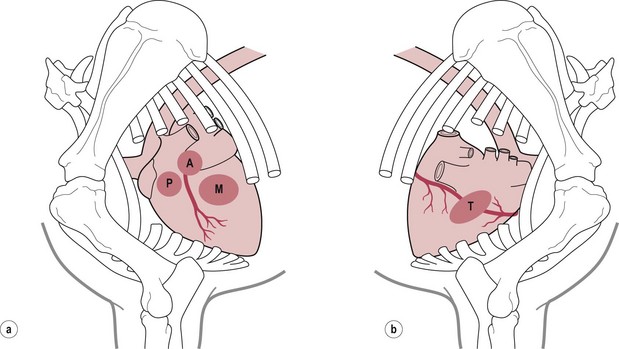
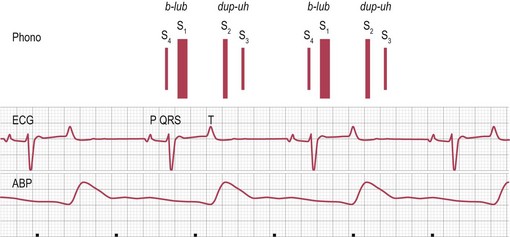
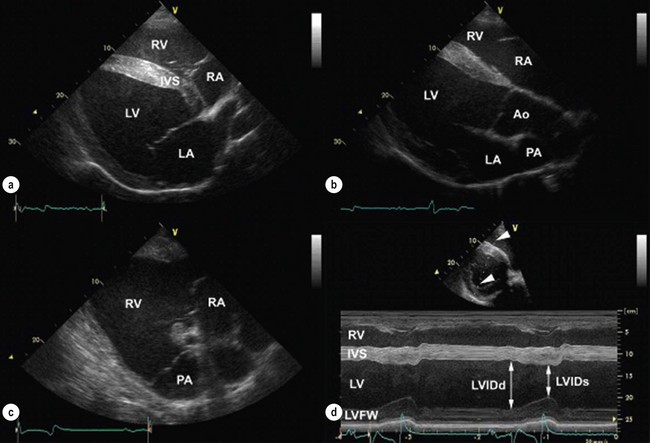
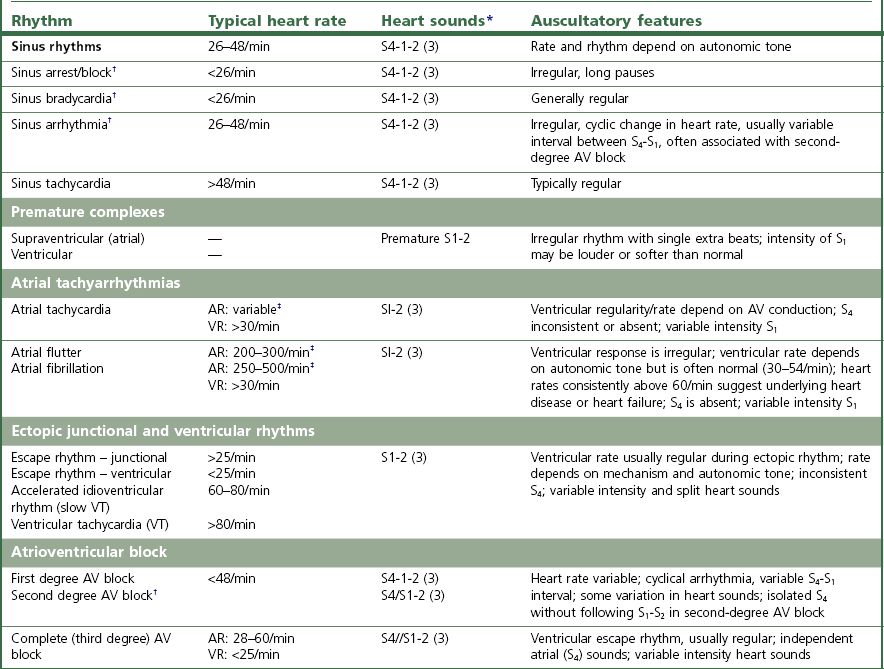
Chapter 7 • Generally (and with exceptions), congenital defects are present from birth and are diagnosed in foals and young horses (<3–5 years). Acquired diseases usually develop with advancing age and are more likely diagnosed in middle-aged and old horses. • Congenital defects may be more common in certain breeds (e.g. Arabians). • Some diseases may depend on the sex of the horse (e.g. aorto-cardiac fistulas in stallions). • The current and prospective use of the horse, the current exercise capacity, and the actual and expected level of performance are important for assessment of the clinical relevance and prognosis of cardiac disease. • Previous health issues may be a cause or a result of cardiovascular disease and should be carefully reviewed. • Presenting complaints are important indicators of the severity and the clinical relevance of cardiovascular disease. • An increase in body surface temperature may indicate fever secondary to an infectious or inflammatory process (e.g. endocarditis or pericarditis); conversely, a cool or cold body surface may be due to poor cardiac output, hypotension, intense peripheral vasoconstriction, and circulatory collapse. • Reduced skin turgor (>1 second) may indicate dehydration. • The mucous membranes may be pale in patients with anaemia or intense peripheral vasoconstriction. Injected (dark red) mucous membranes are seen in association with septicaemia or endotoxaemia. Grey mucous membranes may indicate circulatory collapse and poor peripheral perfusion. Cyanosis (bluish discoloration due to severe hypoxia) is a rare finding in horses with cardiac disease. • The capillary refill time (CRT) is prolonged (>2 seconds) during hypotension and with hypovolaemia, poor cardiac output, or intense peripheral vasoconstriction; conversely, it may be shortened (<1 second) when there is vasodilation. • Jugular pulsations may be observed in healthy horses in the ventral one third of the neck and are more pronounced in excited horses or in horses with very high sympathetic tone. Abnormal jugular pulses may be observed with arrhythmias, with diseases of the tricuspid valve, with pericardial diseases, and with right ventricular or biventricular failure. • Jugular vein distension can be caused by obstruction to flow (e.g. jugular vein thrombosis, intrathoracic mass), pericardial disease, increased intrathoracic pressure (e.g. pleural effusion), or heart failure. However, many horses have significant heart disease without jugular vein distension. A lack of appropriate jugular vein filling is consistent with severe hypovolaemia or venous occlusion. • Heart rate and rhythm as well as altered haemodynamic states can be identified by palpating the facial artery pulse. The arterial pulse can be described as normal, hypokinetic (weak), hyperkinetic (bounding or ‘water-hammer’ pulse), or variable. Irregularity and variable pulse quality often result from cardiac arrhythmia. • Peripheral oedema may indicate right ventricular or biventricular failure, vascular occlusion, severe hypoproteinaemia, vascular disease, or impaired lymphatic drainage. The latter causes should be ruled out even if there is evidence of heart disease. • Nasal discharge, rapid breathing, increased depth of breathing, or increased respiratory effort at rest or following exercise may indicate cardiovascular or respiratory disease. Cough is relatively uncommon in horses with left-heart failure and more likely indicates respiratory disease. • Normal cardiac auscultation in a horse with good exercise tolerance practically precludes clinically relevant heart disease. • Most sustained or recurrent cardiac arrhythmias and most clinically relevant heart murmurs are easily discovered through cardiac auscultation. • Crackles or wheezes on thoracic auscultation may indicate left-heart failure or respiratory disease. Auscultation should be performed in a quiet environment, using a high-quality stethoscope. Knowledge of the areas for auscultation (Figure 7.1) and appreciation of normal heart sounds in the horse (Figure 7.2) are important prerequisites. Also, the examiner must be familiar with the causes and clinical features of murmurs and arrhythmias in the horse (Tables 7.1 and 7.2) and needs to be able to precisely describe the auscultatory findings. Table 7.1 Auscultation of cardiac arrhythmias *( ) = may be evident; †usually physiological; ‡exact rate limits are not established in horses; AR = atrial rate; VR = ventricular rate; AV = atrio-ventricular; VT = ventricular tachycardia. Published in Schwarzwald CC, Bonagura JD, Muir WW (2009) The cardiovascular system. In: Muir WW (ed) Equine anesthesia – monitoring and emergency therapy, 2nd edn, Saunders, p 66, reprinted with permission of Elsevier Ltd. Table 7.2 Identification of heart sounds and common cardiac murmurs °S – systole, the interval between S1 and S2; D – diastole, the interval between S2 and S1. *Only typical features are considered; ‘apex’ refers to the ventral part of the heart, at the point of the palpable cardiac impulse (apical beat); ‘base’ refers to the craniodorsal part of the heart over the outlet valves (aortic, pulmonic), where the second heart sound is most intense. †Functional murmurs are typically soft, localized, and labile. ‡Ventricular inlets refer to the parts of the thorax overlying the ventricular inflow tracts. These include the areas just dorsal to the mitral and tricuspid valve areas and extend ventrally to the apical regions of the ventricles. §Murmurs of AV valve insufficiency are generally heard over the affected valve, project prominently toward the respective ventricular apex, and also radiate dorsally, following the regurgitant jet into the atrium. Late systolic murmurs, which may be related to valve prolapse, have been identified with mitral or tricuspid valve insufficiency. The murmur of aortic insufficiency may not always be holodiastolic. Pulmonary insufficiency is often silent. ||Defects in the RV outlet septum (subpulmonic VSDs, rare) or increased flow across the pulmonic valve (relative pulmonic stenosis) can cause left-basilar systolic murmurs. Flow across very large nonrestrictive septal defects can be relatively soft. From Schwarzwald CC, Bonagura JD, Muir WW (2009) The cardiovascular system. In: Muir WW (ed) Equine anesthesia – monitoring and emergency therapy, 2nd edn, Saunders, p 65. Heart sounds are produced by oscillations of cardio-haemic structures that occur during the cardiac cycle. Their intensity and character depend on loading conditions, rate and force of cardiac contraction, and chamber stiffness. In normal horses, two, three or four heart sounds may be identified on auscultation (Figure 7.2 and Table 7.2). • The atrial sound (S4) is caused by atrial contraction at the end of diastole. It is heard loudest over the heart base and is audible (more or less pronounced) in most normal horses. Variation in the electrical activation of the atria and AV node often leads to gradual changes in the S4–S1 interval. • The systolic heart sound (S1) is associated with ventricular contraction and occurs immediately after closure of the atrioventricular (mitral, tricuspid) valves. It is loudest over the apical area and can be heard in all normal horses. Variations in the intensity and quality of S1 can be caused by changes in electrical activation patterns, after prolonged diastolic periods, and with arrhythmias. • The diastolic heart sound (S2) is associated with the beginning of ventricular relaxation and occurs immediately after closure of the semilunar (aortic, pulmonic) valves. It is loudest over the base of the heart. The second heart sound may be soft or absent after premature beats, when the stroke volume is very small. Close splitting of S2 may occur in normal horses due to asynchronous closure of the aortic and pulmonic valves. Splitting may vary with heart rate and respiration. Splitting of S2 with a loud, accentuated pulmonic component or occurrence of a single loud, pounding S2 may indicate the presence of increased blood pressures in the pulmonary circulation (pulmonary hypertension). • The diastolic filling sound (S3) is caused by rapid inflow and sudden deceleration of blood in the ventricles during early diastole. It is characterized by a low intensity and low frequency and is best heard over the apical area of the heart. The third heart sound may only be heard in a localized area. It may summate with S4 at high heart rates. The third heart sound is considered a normal finding in horses, especially in young and athletic horses. However, S3 may become more pronounced and loud with ventricular dilation and elevated filling pressures (heart failure) and may then be used as a marker of severity of cardiac disease. The examiner needs to be familiar with the auscultatory findings in the normal horse. • The normal resting heart rate generally ranges between 26 and 48/min (Table 7.1). Heart rate in the horse can change rapidly and dramatically, depending on autonomic tone and the level of physical activity. Anxiety and exercise may cause sudden increases in heart rate. • With a normal, regular rhythm, the intensity of heart sounds is steady. Accentuated sounds may be heard with excitement and high sympathetic activity. • The resting normal horse with high vagal tone may exhibit physiologic bradyarrhythmias including atrioventricular block, sinus arrhythmia, and sinus block/arrest (Table 7.1). These arrhythmias are characterized by a slow heart rate (often less than 30/minute) and occasional pauses (‘dropped beats’) that may occur at more or less regular intervals (e.g. one pause after every 4 normal beats). Reducing vagal tone, e.g. by turning the horse quickly in a tight circle or by examining the horse immediately after exercise usually causes the arrhythmia to abate. If the arrhythmia persists or if the auscultatory findings suggest another arrhythmia, an electrocardiogram should be obtained. • Heart murmurs are commonly heard in many normal horses. Most of them are so called functional (flow) murmurs (Table 7.2). Functional murmurs are very common in foals, trained athletes (athletic murmur), and in horses with high sympathetic nervous system activity. They can also be associated with fever and are often heard in anemic horses. • Trivial or mild regurgitation across right-sided (most common) or left-sided (less common) cardiac valves can be identified in some horses by Doppler echocardiography. This is considered a normal finding of no clinical relevance provided that the regurgitation is of short duration and no other findings indicate the presence of cardiac disease. Many (up to 30%) trained equine athletes have audible murmurs of tricuspid and/or mitral regurgitation that are not associated with poor performance or signs of heart disease (by physical examination and echocardiography) and are not considered clinically relevant. Clinically negligible aortic and pulmonary insufficiencies are often silent. • The continuous murmur of patent ductus arteriosus is normally present in full-term foals for up to 4 days post parturition. Furthermore, normal foals can present with soft, left-basilar functional murmurs that are heard during systole and that can persist up to 30–60 days after birth. 1. Timing and duration. Murmurs can be systolic (from S1 to S2), diastolic (from S2 to S1), or continuous (Figure 7.2, Table 7.2). Depending on the timing of appearance they are called early, mid, or late systolic and diastolic, respectively. Murmurs that are heard throughout systole and diastolic are referred to as holosystolic and holodiastolic. 2. Grade. Grade 1/6 murmurs are very quiet, are only heard over a very localized area after careful auscultation in quiet environment, and may be inconsistent. Grade 2/6 murmurs are quiet, but are heard consistently over the point of maximal intensity. Grade 3/6 murmurs are moderately loud, are heard immediately and consistently, and have a small area of radiation. Grade 4/6 murmurs are loud, radiate over a wider area, but are not associated with a palpable thrill. Grade 5/6 murmurs are very loud, radiate over a widespread area, and are associated with a palpable thrill. Grade 6/6 murmurs are extremely loud and are also heard with the stethoscope held just above the skin surface. 3. Point of maximal intensity (PMI). Apical murmurs are heard best at the location of the thoracic wall cardiac impulse (apical beat), approximately at or slightly above the level of the elbow (ventral region of the left ventricular inlet). Basilar murmurs are heard best over the area above the elbow and slightly more cranial, below the triceps muscle (region of the ventricular outflow tracts, semilunar valves, and great vessels). The PMI can also be described in relation to the pulmonic, aortic, mitral, and tricuspid valve areas (Figure 7.1). 4. Radiation. Murmurs can radiate dorsally or ventrally, cranially or caudally, and to the left or right in relation to the PMI. 5. Quality. The quality of the murmurs can be characterized based on the frequency of the sound as high pitch, low pitch, or mixed pitch. Furthermore, murmurs can be described as harsh, coarse, rumbling, muscial, squeaky, honking, or blowing. • Besides the heart sounds and classical murmurs, ‘extra sounds’ are sometimes detected. Electrocardiography is the method of choice for assessment of cardiac rhythm disturbances. • A resting electrocardiogram (ECG) recorded over a few minutes or less may reveal frequent or persistent rhythm disturbances, while infrequent or episodic disturbances may go undetected. • Telemetric ECG recorders can be used in exercising horses and allow detection of exercise-induced arrhythmias. • Ambulatory (Holter) ECG equipment is used for rhythm monitoring over a prolonged period of time (up to 24 hours or longer) and allows detection of infrequent or episodic arrhythmias. • Generally, a normal ECG does not preclude heart disease in the horse, and the ECG cannot be used to assess cardiac dimensions or mechanical function of the myocardium. The electrocardiogram is a tracing of the average electric potential generated by the heart muscle recorded throughout the different phases of the cardiac activation process and graphed in terms of voltage (displayed on the y axis) and time (displayed along the x axis) (Figure 7.2). • The P wave is generated by atrial depolarization, originating from the sino-atrial node. • The PQ interval (often also referred to as PR interval) represents the time for conduction across the atrio-ventricular node and the His-Purkinje system. • The QRS complex represents the electrical activity during ventricular excitation (depolarization). • Repolarization of the ventricles begins at the end of the QRS complex and extends to the end of the T wave. • The QT interval represents the total electrical activation-repolarization time. • The amplitude and duration of these waveforms depend on many factors, including body mass and age of the horse, lead examined, size of the cardiac chambers, and mode of electrical activation. A systematic approach to ECG analysis should be undertaken. • The base–apex lead is the preferred lead for monitoring cardiac rhythm in horses. To obtain a base–apex recording, the left arm (LA) electrode is placed left over the cardiac apex (left chest, just caudal to the olecranon), the right arm (RA) electrode is placed over the right jugular furrow, and ‘lead I’ is selected on the electrocardiograph. The left leg (LL) electrode can be placed anywhere on the horse; for convenience, it may be placed next to the LA electrode on the left side of the chest. • A paper speed of 25 mm/sec and voltage calibration of 1 cm/mV are commonly used for standard ECGs. These settings may need to be adjusted to obtain a good quality tracing. • Evaluation of the electrocardiogram includes: 1. Determination of heart rate. • Ventricular rate (count QRS-T complexes) • The normal resting heart rate in adult horses ranges between 26 and 48 beats/min. Small-breed horses and ponies may have slightly higher resting heart rates (up to 54 beats/min). • In normal horses, atrial rates should equal ventricular rates unless second-degree AV block is present (see below). • Based on the heart rate, arrhythmias can be categorized as tachyarrhythmias (elevated heart rate) or bradyarrhythmias (slow heart rate). 2. Assessment of cardiac rhythm. • Regular rhythm. RR intervals do not vary significantly or change abruptly. • Regularly irregular rhythm. Patterns of repeating cycles can be identified (e.g. second-degree AV block with 4 normal beats followed by 1 blocked beat). • Irregularly irregular rhythm. Chaotic rhythm without any pattern (i.e. RR intervals vary abruptly and in a random fashion; e.g. atrial fibrillation with irregular atrio-ventricular impulse conduction or multiform ventricular tachycardia). 3. Assessment of waves and complexes. • Identify and compare normal and abnormal complexes. Even the most severe, persistent arrhythmias may be interspersed with normal P-QRS-T complexes. Identification of these complexes may greatly facilitate the assessment of abnormal complexes and is often a prerequisite for a correct rhythm diagnosis. • Assess the morphology, variations, and associations of waves and complexes. P wave. The normal P wave is positive (when using a standard base-apex lead) and can be notched or bifid; however, single-peaked, biphasic (negative/positive), and polyphasic P waves may be encountered. The P wave morphology can change in a cyclic manner during sinus arrhythmia in horses (wandering pacemaker). During normal sinus rhythm, every P wave should be followed by a QRS complex. Second-degree AV block is seen frequently in healthy horses, in which case some of the P waves are not followed by a QRS complex. PQ interval. Physiological variation in the PQ interval can be observed in many horses. Values that persistently exceed 450–500 ms are considered abnormal in horses of larger breeds and indicate disturbed atrio-ventricular impulse conduction (i.e. first-degree AV block). The PQ interval is shorter in smaller horses and ponies, with an upper limit of approximately 300–350 ms. QRS complex. In normal horses, all QRS complexes are morphologically identical and are preceded by a normal P wave. When using a standard base-apex lead, normal QRS complexes appear negative. The normal duration of the QRS complex is 80–140 ms in large-breed horses and 60–120 ms in smaller horses. Abnormal conformation (i.e. duration, orientation) of the QRS complex indicates ventricular conduction disturbance (in which case the P wave precedes the QRS complex, indicating a supraventricular activation pathway) or activation of the ventricles from an ectopic ventricular focus (in which case a preceding P wave cannot be identified). T wave. The T wave morphology is variable in horses. Using a standard base–apex lead, the T wave is usually biphasic (negative/positive). Physiological changes in morphology or size occur during exercise (e.g. large, positive T waves). Enlargement of the T wave may also develop with myocardial hypoxia or hyperkalaemia (see below). Furthermore, altered T waves can be seen following abnormal QRS complexes (secondary T wave changes after abnormal ventricular activation). QT interval. The upper limits for the QT interval at resting heart rates are approximately 560–600 ms in adult horses. The QT interval shortens at higher heart rates and strongly depends on changes in autonomic tone. Prolongation of the QT interval indicates delayed ventricular repolarization and can predispose to ventricular arrhythmias. It may be caused by a variety of drugs (e.g. quinidine, procainamide, flecainide, macrolide antibiotics). • Determine the site of abnormal impulse formation: Supraventricular arrhythmias originate above the AV node. They are characterized by a normal QRS-T complex that is preceded by a P wave or by flutter/fibrillation waves (see below). Junctional arrhythmias originate in the AV node or bundle of His. They are characterized by a normal or near-normal QRS-T morphology. P waves may be identified, but are not consistently associated with the QRS complexes (AV dissociation; see below). Ventricular arrhythmias originate below the bundle of His. They are characterized by an abnormal (bizarre) QRS-T morphology (i.e. wide, large complexes that are oriented differently compared to normal complexes). P waves may be identified between the abnormal complexes, but are not consistently associated with the QRS complexes (AV dissociation; see below). The most important indications for echocardiography in horses include: • Evaluation of heart murmurs. Identification of the source of the murmur, differentiation between physiological and pathological murmurs, assessment of the effects on heart size and function, and assessment of the clinical relevance of pathological murmurs. • Evaluation of cardiac arrhythmias. Detection of underlying structural or functional cardiac disease (e.g. mitral regurgitation and left atrial dilation leading to atrial fibrillation). • Evaluation of suspected congenital defects. Evaluation of heart murmurs, unexplained cyanosis, dysrhythmias, or signs of heart failure in neonates. • Evaluation of congestive heart failure. Identification of the cause of heart failure, assessment of severity of underlying disease, monitoring of progression and response to treatment. • Evaluation of severe respiratory disease: Diagnosis of pulmonary hypertension or Cor pulmonale, detection of patent foramen ovale in foals with respiratory disease. • Two-dimensional echocardiography allows evaluation of the size of the cardiac chambers and the large vessels (aorta, pulmonary artery), the structure and thickness of the chamber walls, the structure and function of the heart valves, the myocardial function, and the anatomical relationship between the cardiac chambers and the large vessels. • M-mode echocardiography shows the cardiac structures on the cursor line displayed over time. The motion of the heart and heart valves can be recorded with a very high temporal resolution. M-mode is routinely used for measurement of the left ventricular diameter, left ventricular free wall, and interventricular septal thickness in systole and diastole. The fractional shortening, an indicator of left ventricular systolic function, can be calculated. • Doppler echocardiography is used to estimate blood flow velocities using the shift in ultrasound frequency which occurs after the ultrasound waves have been reflected by moving red blood cells (Doppler principle). Figure 7.3 shows echocardiograms obtained from the right side of the chest. • Evaluation during and after exercise may be useful in asymptomatic horses or in horses with unspecific or subtle clinical signs (e.g. exercise intolerance) when cardiac disease is suspected. • Horses can be lunged, ridden, or exercised on a high-speed treadmill. The level of exercise should be chosen individually according to the horses’ condition and training status. • Heart rate monitors or telemetric ECG units can be used allowing heart rate and rhythm to be monitored before, during, and after exercise. If such equipment is not readily available, heart rate and rhythm can be obtained by auscultation immediately after exercise. Post-exercise recovery should be monitored every 5 min for at least 20 min or until heart rate and respiratory rates are normal. • As a general rule, the heart rates in fit horses range between 60 and 80 beats/min during walking, 80–120 beats/min during trotting, 120–150 beats/min at a moderate canter, 150–180 beats/min at a gallop, and up to 200–240 beats/min at maximal effort. The heart rate in fit horses falls quickly in the first minute after exercise, should be below 100 beats/min after 4–5 min (usually after 1–2 min), and reaches baseline values within 15–30 min of strenuous exercise. Recovery times are strongly influenced by the fitness of the horse, the level of exercise, and environmental factors. Cardiovascular or respiratory dysfunction can increase heart rates during exercise and may prolong recovery times. • Respiratory rate should not exceed heart rates at any time during exercise and should return to baseline values within 10–20 min after exercise. • Transient sinus arrhythmia and second-degree AV block are considered normal findings in the immediate post-exercise period. Occasional, isolated supraventricular or ventricular ectopic beats can be seen in many normal horses during and after exercise and are usually not of a concern. However, persistent or more severe cardiac rhythm disturbances indicate cardiac disease and warrant further investigation. • Complete blood count and plasma fibrinogen concentration serve to identify anaemia and signs of inflammation that may indicate inflammatory or infectious cardiovascular disease (e.g. endocarditis, pericarditis, thrombophlebitis). • Serum electrolyte concentrations aid in the assessment of cardiac arrhythmias that can be caused by or associated with electrolyte disturbances (e.g. hypo- or hyperkalaemia, hypomagnesaemia). Assessment of fractional excretion of electrolytes in the urine may provide additional information on electrolyte homeostasis. • Indices of renal function (blood urea nitrogen and creatinine concentrations) are important for assessment of volume status and detection of impaired renal perfusion in patients with compromised cardiovascular function. • Elevated serum concentrations of unspecific muscle enzymes (creatine kinase [CK], aspartate transaminase [AST]) and cardiac-specific enzymes (cardiac troponin T [cTnT], cardiac troponin I [cTnI]) allow diagnosis of myocardial cell injury. • Blood lactate concentration as well as arterial and venous blood gas analyses, respectively, serve to evaluate arterial oxygenation and pulmonary function, acid-base status, and – with limitations – oxygen delivery and oxygen utilization in the tissues. • Arterial blood gas analyses may also be useful in the diagnosis of intracardiac shunts. A normal partial pressure of oxygen in arterial blood (paO2) should be >65 mmHg in foals 24 hours post natum and >90 mmHg in older foals and in adults. Hypoxaemia can be caused by right-to-left shunting across a cardiac or vascular defect, pulmonary hypoperfusion, or respiratory disease. Intranasal supplementation of oxygen will not increase the paO2 in horses with shunts, but may improve arterial oxygenation in horses with respiratory disease. • Pericardial effusates should be collected and submitted for cytological examination, bacterial cultures, and antibiotic sensitivity testing. • Blood cultures are recommended in cases of thrombophlebitis or suspected endocarditis to diagnose bacteraemia, identify the aetiologic agents, and determine the antibiotic sensitivity pattern. • Monitoring of serum or plasma drug concentrations are indicated when certain drugs (e.g. quinidine, digoxin) are used to treat patients with heart disease. • Arterial blood pressure can be measured invasively (by means of arterial catheterization) or non-invasively (using a variety of auscultatory, Doppler, or oscillometric techniques). • Blood pressure monitoring is a routine procedure during general anaesthesia in horses. Furthermore, blood pressure measurement is commonly used for assessment of cardiovascular function in critically ill neonates. • Arterial blood pressure monitoring includes measurement of systolic, diastolic, and mean pressures. • Arterial pressure depends on the interplay between cardiac output and vascular resistance. Therefore, arterial pressure is not a reliable index of blood flow if vascular resistance (vascular tone) is abnormal or is changing over time. • Normal reported values for indirect arterial systolic and diastolic pressures are 111.8 ± 13.3 (mean ± SD) and 67.7 ± 13.8, respectively. • The systolic pressure is generated by the left ventricle and is affected by stroke volume, aortic/arterial compliance, and the previous diastolic blood pressure. • Arterial pulse pressure, the difference between systolic and diastolic pressures, is highly dependent on stroke volume and the peripheral arteriolar resistance, which determines the run-off of diastolic pressure. The pulse pressure is the primary determinant of the intensity of the palpable peripheral arterial pulse. • Ventricular failure reduces pulse pressure (hypokinetic, weak pulse), whereas increased stroke volume and abnormal diastolic run-off due to aortic insufficiency or generalized vasodilation widens the pulse pressure (hyperkinetic, bounding, or water-hammer type pulse). • Diastolic and mean pressures are better estimates of perfusion pressure than systolic pressures. • The diagnostic value of radiography in horses with cardiovascular disease is limited. • While gross cardiac enlargement can be detected on thoracic radiographs, echocardiography is better suited for accurate measurement of chamber size, assessment of chamber function, and detection of structural cardiac or vascular lesions. • Thoracic radiographs are primarily used to assess pulmonary structures and can be used in the differential diagnosis of respiratory disease and for detection of cardiogenic pulmonary congestion or oedema, respectively. • Angiographic methods (i.e. contrast radiography and contrast computed tomography) and in the future also cardiac magnetic resonance imaging may be used to assess heart size and heart function and to diagnose congenital heart disease in foals. However, the application of these methods is generally limited to research or to cases where echocardiography is inconclusive or unavailable. • Cardiac catheterization serves to measure intracardiac pressures, cardiac output, and vascular resistance and to assess oxygenation of blood in the various cardiac chambers. • It can be used to assess central haemodynamics as well as systolic and diastolic cardiac function. Furthermore, the results may demonstrate shunting of blood between the systemic and the pulmonic circulation that may occur in association with congenital cardiac defects. • Cardiac catheterization is rarely used for routine diagnosis of cardiovascular disease in horses and has largely been replaced by Doppler echocardiography for clinical purposes. Its use is generally limited to research applications. • Occasionally, cardiac catheterization is performed in critically ill foals and in selected cases for cardiovascular monitoring during anaesthesia. • Approximately 3% of all horses presented for a cardiovascular workup are diagnosed with congenital heart disease. • Congenital heart disease should be considered when a foal, weanling, or young horse is identified with a prominent cardiac murmur, cyanosis, or signs of congestive heart failure. • Ventricular septal defects result in systemic-to-pulmonary (left-to-right) shunting of blood. The shunt volume and the resulting haemodynamic sequelae depend on the size of the lesion and the resistances of the systemic and the pulmonary circulation, respectively. • The clinical presentation varies depending on the size of the defect and shunt volume. • The typical heart murmur associated with a membranous VSD is a band-shaped, holosystolic murmur heard best over the right side of the chest, just below the tricuspid valve. Murmurs are often prominent (grade ≥3/6) and may be associated with a palpable thrill (grade ≥5/6). • Echocardiographic studies usually confirm the lesion. In combination with work history and physical examination, 2D and Doppler echocardiography further allow assessment of haemodynamic consequences, severity of the malformation, and prognosis.
Cardiovascular system
7.2 Evaluation of cardiovascular function
Signalment
Medical history
 Heart murmurs and cardiac arrhythmias are common in athletic horses and must be evaluated in relation to the medical history and physical examination findings; they may not be clinically relevant in the absence of other clinical signs consistent with heart disease.
Heart murmurs and cardiac arrhythmias are common in athletic horses and must be evaluated in relation to the medical history and physical examination findings; they may not be clinically relevant in the absence of other clinical signs consistent with heart disease.
 Exercise intolerance and poor performance are common, but very unspecific complaints that can be associated with cardiac disease and early heart failure. Other causes of poor performance such as respiratory, orthopaedic, neurological, or metabolic disorders also have to be considered. Generally, normal performance in a top-class athlete likely precludes the presence of severe cardiac disease and heart failure.
Exercise intolerance and poor performance are common, but very unspecific complaints that can be associated with cardiac disease and early heart failure. Other causes of poor performance such as respiratory, orthopaedic, neurological, or metabolic disorders also have to be considered. Generally, normal performance in a top-class athlete likely precludes the presence of severe cardiac disease and heart failure.
 Cough, nasal discharge, and increased respiratory effort may be signs of advanced heart failure or respiratory disease.
Cough, nasal discharge, and increased respiratory effort may be signs of advanced heart failure or respiratory disease.
 Poor growth and exercise intolerance in foals (often associated with apparent signs of respiratory disease) may be a result of cardiac disease.
Poor growth and exercise intolerance in foals (often associated with apparent signs of respiratory disease) may be a result of cardiac disease.
 Horses with severe systemic disease such as patients with gastrointestinal disease, endotoxaemia, septicaemia, or severe acid–base and electrolyte disturbances are likely to suffer from some degree of circulatory compromise. Assessment of the volume status and cardiovascular function is therefore of great importance in all severely ill patients to choose the appropriate protocols for treatment and monitoring.
Horses with severe systemic disease such as patients with gastrointestinal disease, endotoxaemia, septicaemia, or severe acid–base and electrolyte disturbances are likely to suffer from some degree of circulatory compromise. Assessment of the volume status and cardiovascular function is therefore of great importance in all severely ill patients to choose the appropriate protocols for treatment and monitoring.
Physical examination
Inspection and palpation
 It should be noted that palpation of a peripheral pulse wave only allows estimation of pulse pressure (difference between systolic and diastolic arterial blood pressure). However, it neither provides an accurate estimate of absolute systolic and diastolic blood pressures nor corresponds to blood flow or tissue perfusion.
It should be noted that palpation of a peripheral pulse wave only allows estimation of pulse pressure (difference between systolic and diastolic arterial blood pressure). However, it neither provides an accurate estimate of absolute systolic and diastolic blood pressures nor corresponds to blood flow or tissue perfusion.
Auscultation
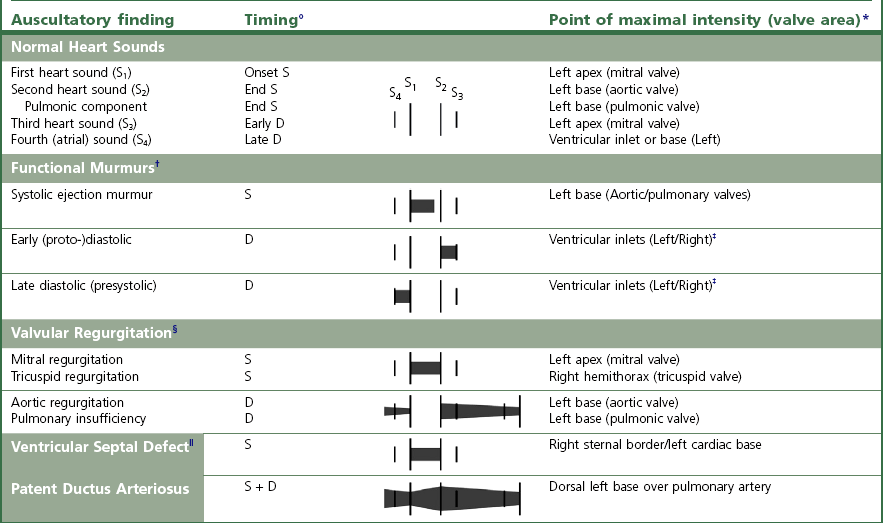
 Typically, functional murmurs are soft (grade 1–3/6), localized, and labile. They are very dependent on physiological state and can be altered by changing the heart rate (e.g. they may become more prominent at higher heart rates).
Typically, functional murmurs are soft (grade 1–3/6), localized, and labile. They are very dependent on physiological state and can be altered by changing the heart rate (e.g. they may become more prominent at higher heart rates).
 The most common physiologic murmur is the systolic ejection murmur which begins after the first heart sound and ends before the second heart sound and is heard best at the left cardiac base over the aortic and pulmonic valves.
The most common physiologic murmur is the systolic ejection murmur which begins after the first heart sound and ends before the second heart sound and is heard best at the left cardiac base over the aortic and pulmonic valves.
 Early to mid diastolic murmurs extend from S2 to S3 and are associated with rapid filling of the ventricles. They are quite common, especially in athletic horses, and may be musical (squeaky) in character.
Early to mid diastolic murmurs extend from S2 to S3 and are associated with rapid filling of the ventricles. They are quite common, especially in athletic horses, and may be musical (squeaky) in character.
 An increase in heart rate (tachycardia) can be due to exercise, excitement, stress and anxiety, pain, fever, hypovolaemia and hypotension, anaemia, shock, metabolic derangements, electrolyte imbalances, drug effects (e.g. atropine, catecholamines), or primary heart disease (e.g. tachyarrhythmias, heart failure).
An increase in heart rate (tachycardia) can be due to exercise, excitement, stress and anxiety, pain, fever, hypovolaemia and hypotension, anaemia, shock, metabolic derangements, electrolyte imbalances, drug effects (e.g. atropine, catecholamines), or primary heart disease (e.g. tachyarrhythmias, heart failure).
 A decrease in heart rate (bradycardia) may result from an increase in vagal tone, drug effects (e.g. alpha-2 agonists, anaesthetics, digitalis glycosides), hypothermia, electrolyte imbalances (e.g. severe hyperkalaemia), or primary disturbances of impulse formation or impulse conduction in the heart.
A decrease in heart rate (bradycardia) may result from an increase in vagal tone, drug effects (e.g. alpha-2 agonists, anaesthetics, digitalis glycosides), hypothermia, electrolyte imbalances (e.g. severe hyperkalaemia), or primary disturbances of impulse formation or impulse conduction in the heart.
 Unexplained, persistent tachycardia or bradycardia should be assessed by means of electrocardiography to rule out rhythm disturbances other than sinus tachycardia and sinus bradycardia, respectively (Table 7.1).
Unexplained, persistent tachycardia or bradycardia should be assessed by means of electrocardiography to rule out rhythm disturbances other than sinus tachycardia and sinus bradycardia, respectively (Table 7.1).
 Accentuated sounds may be heard with volume overload or high sympathetic activity.
Accentuated sounds may be heard with volume overload or high sympathetic activity.
 Varying intensity of normal heart sounds can occur due to cardiac rhythm disturbances leading to irregular ventricular filling and stroke volume.
Varying intensity of normal heart sounds can occur due to cardiac rhythm disturbances leading to irregular ventricular filling and stroke volume.
 Muffled heart sounds can indicate pericarditis and pericardial effusion.
Muffled heart sounds can indicate pericarditis and pericardial effusion.
 With some exceptions (e.g. slow ventricular tachycardia or some ventricular conduction disturbances), cardiac rhythm disturbances are generally easily detected by careful auscultation. Pathological rhythm disturbances need to be differentiated from physiological dysrhythmias (Table 7.1).
With some exceptions (e.g. slow ventricular tachycardia or some ventricular conduction disturbances), cardiac rhythm disturbances are generally easily detected by careful auscultation. Pathological rhythm disturbances need to be differentiated from physiological dysrhythmias (Table 7.1).
 The presence of a cardiac murmur is an essential finding that leads one to suspect potential acquired valvular disease or a congenital cardiac malformation. Pathological murmurs need to be differentiated from physiological murmurs (Table 7.2).
The presence of a cardiac murmur is an essential finding that leads one to suspect potential acquired valvular disease or a congenital cardiac malformation. Pathological murmurs need to be differentiated from physiological murmurs (Table 7.2).
 Heart murmurs should be characterized based on timing and duration, grade, point of maximal intensity, radiation, and quality.
Heart murmurs should be characterized based on timing and duration, grade, point of maximal intensity, radiation, and quality.
 Most murmurs can be distinguished through careful auscultation (Table 7.2), including evaluation of the effect of changing heart rate on the heart murmurs (e.g. by jogging or turning the horse in a tight circle). The assessment of the clinical relevance of an organic heart murmur may require ancillary tests (see below).
Most murmurs can be distinguished through careful auscultation (Table 7.2), including evaluation of the effect of changing heart rate on the heart murmurs (e.g. by jogging or turning the horse in a tight circle). The assessment of the clinical relevance of an organic heart murmur may require ancillary tests (see below).
 Causes of pathological murmurs include incompetent cardiac valves, septal defects, and vascular lesions. Valvular stenosis is very rare in horses and can be disregarded for most instances.
Causes of pathological murmurs include incompetent cardiac valves, septal defects, and vascular lesions. Valvular stenosis is very rare in horses and can be disregarded for most instances.
 Significant myocardial disease may be associated with both arrhythmias and cardiac murmurs, especially in advanced cases in which ventricular dilatation causes secondary insufficiency of the mitral or tricuspid valves.
Significant myocardial disease may be associated with both arrhythmias and cardiac murmurs, especially in advanced cases in which ventricular dilatation causes secondary insufficiency of the mitral or tricuspid valves.
 Systolic clicks can occasionally by heard over the left apex and may indicate mitral valve disease or prolapse (especially when associated with a mid- to end-systolic murmur of mitral regurgitation).
Systolic clicks can occasionally by heard over the left apex and may indicate mitral valve disease or prolapse (especially when associated with a mid- to end-systolic murmur of mitral regurgitation).
 Ventricular knocks are loud ventricular filling sounds (correlating to S3) heard with constrictive pericardial disease.
Ventricular knocks are loud ventricular filling sounds (correlating to S3) heard with constrictive pericardial disease.
 Pericardial friction rub typically is a triphasic creaking or scratching sound (systole, early diastole, late diastole) indicating pericarditis.
Pericardial friction rub typically is a triphasic creaking or scratching sound (systole, early diastole, late diastole) indicating pericarditis.
Ancillary diagnostics
Echocardiography
 A normal echocardiogram in a horse with a cardiac murmur is a favourable finding. Conversely, identification of significant cardiomegaly or abnormal ventricular function may indicate considerable prognostic risk.
A normal echocardiogram in a horse with a cardiac murmur is a favourable finding. Conversely, identification of significant cardiomegaly or abnormal ventricular function may indicate considerable prognostic risk.
 Cardiac disease in horses with exercise intolerance or poor performance.
Cardiac disease in horses with exercise intolerance or poor performance.
 Pericardial disease in horses with muffled heart sounds, pericardial friction rubs, and/or distended jugular veins.
Pericardial disease in horses with muffled heart sounds, pericardial friction rubs, and/or distended jugular veins.
 Endocarditis in horses with fever of unknown origin (± heart murmur).
Endocarditis in horses with fever of unknown origin (± heart murmur).
 Cardiac disease in horses with unexplained collapse or episodic weakness.
Cardiac disease in horses with unexplained collapse or episodic weakness.
 Color flow Doppler is routinely used to screen for disturbances of normal blood flow in a specified area of interest (e.g. around a valve or in areas with congenital defects). Direction of blood flow, its velocity, and its flow characteristics (e.g. turbulent flow vs. laminar flow) can be assessed using a colour-coded display superimposed to a 2D image (for location) or M-mode recording (for timing). Traditionally, flow directed toward the transducer is coded red and flow away from the transducer is coded blue. Turbulent flow may be coloured green.
Color flow Doppler is routinely used to screen for disturbances of normal blood flow in a specified area of interest (e.g. around a valve or in areas with congenital defects). Direction of blood flow, its velocity, and its flow characteristics (e.g. turbulent flow vs. laminar flow) can be assessed using a colour-coded display superimposed to a 2D image (for location) or M-mode recording (for timing). Traditionally, flow directed toward the transducer is coded red and flow away from the transducer is coded blue. Turbulent flow may be coloured green.
 In pulsed-wave (PW) Doppler and continuous-wave (CW) Doppler modes, blood flow velocity is displayed (on the y-axis) versus time (on the x-axis). Pressure gradients between two chambers (e.g. between the left and right ventricle in the presence of a VSD, or between the right atrium and the right ventricle in the presence of tricuspid regurgitation) can be estimated based on the velocity of blood flowing between the two chambers measured by Doppler echocardiography. Estimation of pressure gradients is a very useful tool for evaluation of hemodynamic consequences of cardiac disease. For example, Doppler echocardiography can be used for assessment of haemodynamic significance of ventricular septal defects (pressure difference between right and left ventricle), or for detection of pulmonary hypertension (if tricuspid regurgitation or pulmonic insufficiency are present).
In pulsed-wave (PW) Doppler and continuous-wave (CW) Doppler modes, blood flow velocity is displayed (on the y-axis) versus time (on the x-axis). Pressure gradients between two chambers (e.g. between the left and right ventricle in the presence of a VSD, or between the right atrium and the right ventricle in the presence of tricuspid regurgitation) can be estimated based on the velocity of blood flowing between the two chambers measured by Doppler echocardiography. Estimation of pressure gradients is a very useful tool for evaluation of hemodynamic consequences of cardiac disease. For example, Doppler echocardiography can be used for assessment of haemodynamic significance of ventricular septal defects (pressure difference between right and left ventricle), or for detection of pulmonary hypertension (if tricuspid regurgitation or pulmonic insufficiency are present).
Exercise testing
Clinical laboratory tests
Arterial blood pressure
Radiography
Cardiac catheterization
7.3 Structural heart disease
 Ventricular septal defects (VSD) are most frequently seen. They are usually located in the membranous part of the ventricular septum, just below the aortic and the tricuspid valve (paramembranous VSD). Rarely, VSDs are located in the subpulmonic position (subpulmonic VSD) or in the muscular septum (muscular VSD). VSDs can also be part of complex malformations.
Ventricular septal defects (VSD) are most frequently seen. They are usually located in the membranous part of the ventricular septum, just below the aortic and the tricuspid valve (paramembranous VSD). Rarely, VSDs are located in the subpulmonic position (subpulmonic VSD) or in the muscular septum (muscular VSD). VSDs can also be part of complex malformations.
 Patent ductus arteriosus (PDA) is uncommon in foals and is detected most frequently in combination with complex defects. However, functional closure of the ductus arteriosus may not occur until 72 to 96 hours after birth, resulting in a murmur that can be heard up to an age of four days.
Patent ductus arteriosus (PDA) is uncommon in foals and is detected most frequently in combination with complex defects. However, functional closure of the ductus arteriosus may not occur until 72 to 96 hours after birth, resulting in a murmur that can be heard up to an age of four days.
 Other congenital defects (e.g. atrial septal defect, patent foramen ovale, valvular dysplasia or atresia) are relatively rare. Complex congenital defects usually lead to fetal death, birth of a non-viable foal, or rapid deterioration early after birth.
Other congenital defects (e.g. atrial septal defect, patent foramen ovale, valvular dysplasia or atresia) are relatively rare. Complex congenital defects usually lead to fetal death, birth of a non-viable foal, or rapid deterioration early after birth.
Ventricular septal defect (VSD)
 Immediately after birth, pulmonary vascular resistance is still high and systemic pressures are low, limiting left-to-right shunting. Over the first few weeks of life, significant shunting may develop due to a gradual decline in pulmonary vascular resistance and rise in systemic pressures.
Immediately after birth, pulmonary vascular resistance is still high and systemic pressures are low, limiting left-to-right shunting. Over the first few weeks of life, significant shunting may develop due to a gradual decline in pulmonary vascular resistance and rise in systemic pressures.
 Left-to-right shunt increases pulmonary blood flow and pulmonary venous return to the left heart and results in compensatory left atrial and left ventricular enlargement. With large shunt volumes, left-sided volume overload may be severe, leading to left-sided or biventricular congestive heart failure.
Left-to-right shunt increases pulmonary blood flow and pulmonary venous return to the left heart and results in compensatory left atrial and left ventricular enlargement. With large shunt volumes, left-sided volume overload may be severe, leading to left-sided or biventricular congestive heart failure.
 Pulmonary hypertension can occur due to increased transpulmonary flow and flow-related pulmonary vascular changes. A rise in pulmonary vascular and right ventricular pressures decreases the shunt volume and protects the left heart from severe volume overload while imposing an increased work load on the right ventricle. Rarely, severe pulmonary hypertension may result in reversed (right-to-left) shunt with development of arterial hypoxemia (Eisenmenger’s physiology).
Pulmonary hypertension can occur due to increased transpulmonary flow and flow-related pulmonary vascular changes. A rise in pulmonary vascular and right ventricular pressures decreases the shunt volume and protects the left heart from severe volume overload while imposing an increased work load on the right ventricle. Rarely, severe pulmonary hypertension may result in reversed (right-to-left) shunt with development of arterial hypoxemia (Eisenmenger’s physiology).
 Foals and adults with small defects are often asymptomatic. Murmurs may be detected incidentally during examination for another problem or during prepurchase examination.
Foals and adults with small defects are often asymptomatic. Murmurs may be detected incidentally during examination for another problem or during prepurchase examination.
 Some horses may be asymptomatic at rest but may show signs of exercise intolerance or poor performance at the time when they enter training.
Some horses may be asymptomatic at rest but may show signs of exercise intolerance or poor performance at the time when they enter training.
 Foals with large defects may die early in life or may present with signs of poor growth or congestive heart failure (often misinterpreted as signs of pneumonia) at the age of a few weeks or months.
Foals with large defects may die early in life or may present with signs of poor growth or congestive heart failure (often misinterpreted as signs of pneumonia) at the age of a few weeks or months.
 Cyanosis is not a typical feature of VSDs, because the shunt direction is usually left-to-right. If cyanosis is detected, complex malformations or shunt reversal should be considered.
Cyanosis is not a typical feature of VSDs, because the shunt direction is usually left-to-right. If cyanosis is detected, complex malformations or shunt reversal should be considered.
 The grade of the murmur does not correlate with the size of the defect or the shunt volume.
The grade of the murmur does not correlate with the size of the defect or the shunt volume.
 Other murmurs that may be heard are left-sided systolic murmurs of relative pulmonic stenosis (functional ejections murmurs), left-sided systolic murmurs of mitral regurgitation (due to mitral insufficiency secondary to severe left-ventricular volume overload), or left-sided diastolic murmurs of aortic insufficiency (due to involvement of the aortic valve annulus, resulting in valve prolapse).
Other murmurs that may be heard are left-sided systolic murmurs of relative pulmonic stenosis (functional ejections murmurs), left-sided systolic murmurs of mitral regurgitation (due to mitral insufficiency secondary to severe left-ventricular volume overload), or left-sided diastolic murmurs of aortic insufficiency (due to involvement of the aortic valve annulus, resulting in valve prolapse).
 Ventricular septal defects are termed restrictive when their diameter is less than one third of the diameter of the aorta (<2.5 cm in large-breed adult horses) and the peak velocity of the shunt flow is higher than 4 m/s (corresponding to a left-to-right pressure gradient of at least 64 mmHg).
Ventricular septal defects are termed restrictive when their diameter is less than one third of the diameter of the aorta (<2.5 cm in large-breed adult horses) and the peak velocity of the shunt flow is higher than 4 m/s (corresponding to a left-to-right pressure gradient of at least 64 mmHg).
 Restrictive VSDs are usually well tolerated and have a favourable prognosis for life and potentially even for athletic performance.
Restrictive VSDs are usually well tolerated and have a favourable prognosis for life and potentially even for athletic performance.
 Horses with an excellent work history are unlikely to have a large defect.
Horses with an excellent work history are unlikely to have a large defect.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree