CHAPTER 20 Cardiovascular Diseases
Prevalence and Risk Factors
Prevalence
The prevalence of cardiac disease in the general feline population is not currently determined. Stalis and coworkers83 found that myopathic heart disease was identified in approximately 9% of 1472 feline necropsies from 1986 to 1992 at the University of Pennsylvania. More recently, two small studies (approximately 200 cats in total) have examined the prevalence of cardiac disease in apparently healthy cats.18,61 Côté and colleagues18 examined the prevalence of heart murmurs in apparently healthy cats and detected murmurs in 22 of 103 cats examined. Of these 22 cats, seven had echocardiographic evaluations, and six were considered to have evidence of myocardial hypertrophy (one was normal). Paige and coworkers61 examined 103 apparently healthy cats: 16 of 103 had murmurs, and five of these had evidence of myocardial hypertrophy. Additionally, 11 of 103 cats had evidence of myocardial hypertrophy but no murmurs.
Relative prevalence of cardiac disease has been examined by Harpster31 at a single referral institution. Of 500 cats presenting to the cardiology department at Angell Memorial Animal Hospital from 1987 to 1989, 22% had hypertrophic cardiomyopathy, 15% had unclassified cardiomyopathy, 14% had mitral valve disease, 12% had dilated cardiomyopathy, 10% had thyrotoxic heart disease, and approximately 7% had congenital diseases. Systemic hypertension was identified in 1%. It should be obvious that these percentages do not represent true prevalence (or incidence) but rather describe the distribution of heart diseases in patients presenting for evaluation of heart disease. Additionally, substantial changes in feline nutrition (namely taurine supplementation) and early detection and management of hyperthyroidism have greatly reduced the percentage of cats presenting with either dilated cardiomyopathy or thyrotoxic heart disease.
Similarly, the prevalence of congenital cardiac diseases in cats is much less comprehensively reported than that of dogs. Frequency of congenital heart disease has not been examined in the last 30 years. Buchanan11 estimated that atrioventricular valve malformations were the most common congenital defect in cats, followed by ventricular septal defect (VSD), endocardial fibroelastosis and patent ductus arteriosus (PDA).
Côté and Jaeger17 examined the incidence of structural heart disease in 106 cats presenting with ventricular arrhythmias. Almost all cats with ventricular tachyarrhythmias had echocardiographic evidence of structural heart disease (102 of 106). Prior studies by Fox and associates26 and Fox and Harpster25 suggested substantially lower incidence rates of ventricular tachyarrhythmias in cats with hypertrophic cardiomyopathy (HCM) (10% to 40%). However, both studies reported all arrhythmias in cats with HCM at much higher rates (25% to 70%).
Risk Factors
Certain risk factors are associated with some feline heart disease. HCM has specific breed predispositions, and at least one identified genetic cause in each of two breeds (Maine Coons and Ragdolls).53,55 Sphynx, Norwegian Forest Cats, American Shorthairs, Scottish Folds, Persians, Siamese, Abyssinians, Himalayans, and Birmans are all breeds considered to be predisposed to cardiomyopathies.23 Whether there are any gender differences in expression of genetic traits or in prevalence of congenital disorders is not well defined.
Taurine deficiency was identified as a major cause of dilated cardiomyopathy in cats in the mid 1980s.64 Subsequent supplementation of commercial diets with taurine has led to the almost complete disappearance of taurine-deficient myocardial failure in cats. However, homemade diets can still occasionally lead to taurine deficiency, resulting in dilated cardiomyopathy.
History and Physical Examination
Murmurs
The physical examination of cats with heart disease is often only modestly revealing. Many cats with cardiac disease have no indicative clinical signs or physical examination findings. In one small study, only 5 of 16 of cats with cardiomyopathy had murmurs at initial examination; 11 had occult disease.61 This number increased to 11 of 16 cats when dynamic murmurs (not necessarily ausculted at the time of examination but provoked during echocardiographic evaluation) were examined. Conversely, many cats with a murmur have no identifiable heart disease. Paige and coworkers61 also identified murmurs in 16 of 103 healthy cats but found cardiac disease in only 5 of these; 11 had no evidence of structural disease.
Dynamic murmurs are common findings in cats with and without heart disease. Paige and coworkers61 identified dynamic murmurs in 28 of 103 apparently healthy cats. Dynamic murmurs change in intensity or appear only after provocation (e.g., fear, aggression). They are generally parasternal murmurs (either right or left) and can be extremely transient, lasting only a few beats in some cats. Rishniw and Thomas71 identified a dynamic right ventricular outflow tract obstruction in 50 cats between 1994 and 1996 that was only occasionally associated with structural heart disease. The most common diseases associated with this physiologic murmur were chronic kidney disease and nasal squamous cell carcinoma (SCC), but these cats were examined in California, where nasal SCC is highly prevalent. Cats younger than 4 years of age with dynamic right ventricular outflow tract obstruction most often had HCM.
Systolic anterior motion of the mitral valve and the associated dynamic left ventricular obstruction account for most of the remainder of identifiable dynamic murmurs in cats. This phenomenon is observed predominantly in cats with HCM but can occasionally be observed in cats without any identifiable structural heart disease. Midventricular obstructions have also been identified in cats with HCM or other feline cardiac diseases49 and might account for some dynamic murmurs in cats. In one study only 36% of provocable murmurs had an identifiable etiology; thus many dynamic murmurs might not have an easily identifiable cause.61
Heart Sounds and Arrhythmias
Arrhythmias occur frequently in cats with heart disease. In one retrospective study, 96% of cats with ventricular tachyarrhythmias had echocardiographic evidence of structural heart disease. Thus auscultation of extrasystoles warrants further investigation. It is, however, more difficult to define sustained tachyarrhythmias in cats presenting to clinicians for physical evaluation. Feline heart rates can easily reach 240 to 260 beats per minute (bpm) in stressful situations and can do so in a matter of seconds. Cats stressed by a hospital visit or because of other systemic disease can have sustained heart rates above 220 bpm.1 The astute clinician should note heart rates from prior visits in regular patients to determine whether the rate is appropriate for that patient. Unexpectedly high heart rates, especially those that deviate from rates obtained at prior visits, might warrant further investigation.
Sinus arrhythmias are uncommon in cats in the hospital environment and have been associated mostly with extracardiac disease.69 However, some healthy young cats can have a mild sinus arrhythmia as an incidental finding. Additionally, most cats exhibit brief periods of sinus arrhythmia during sleep.86
Diagnosis of Feline Heart Disease
Electrocardiography
In eupneic cats the ECG is recorded in right lateral recumbency. However, sternal recumbency alters few electrocardiographic parameters of clinical interest.29,32 Therefore assessment in sternal recumbency in fractious, dyspneic, or fragile patients is acceptable.
Continuous 24-hour ambulatory ECG (Holter) monitoring has historically been less successful in cats than dogs, largely because of the size of the recording systems. New digital Holter systems are small enough to be attached to the cat with adhesive bandaging. Holter monitoring can provide diagnostic information in cats with syncope.22 Additionally, small event recorders can be surgically implanted into syncopal patients to increase the probability of arrhythmia detection.20,38 Holter monitors should not be used on cats with severe structural heart disease or CHF because the stress of monitoring can result in the death of the patient.
Electrocardiography as a Screening Test for Subclinical Heart Disease
ECG is ineffective as a screening tool for occult cardiac disease in cats. The basis for using ECG as a screening tool relies on its ability to detect either chamber enlargement or shifts in the mean electrical axis (MEA). However, ECG is extremely insensitive and relatively imprecise in detecting chamber enlargement (or myocardial concentric hypertrophy), and although it can identify deviations in the MEA, these occur relatively infrequently in the general population and can occur in cats with and without underlying structural disease. Only one study has examined the ability of ECG to identify left atrial enlargement in cats.76 This study showed poor sensitivity (12% to 60%) and good specificity (72% to 100%), suggesting that very few cats with p-wave abnormalities have normal left atria. No equivalent studies exist that specifically examine the sensitivity and specificity of ECG in detecting ventricular enlargement in cats; however, studies in humans and other species suggest sensitivities of approximately 50% and specificities of 80% (similar to those found by Schober and coworkers76 for left atrial enlargement). Two studies have examined ECG abnormalities in cats with heart disease. Ferasin and coworkers21 identified 106 cats with varying degrees of HCM; of these 41 (39%) had no identifiable ECG abnormalities. Riesen and coworkers68 examined 395 cats with various symptomatic heart diseases, including 169 cats with HCM; of these 35 (21%) had no identifiable ECG abnormalities. Riesen and coworkers identified morphologic changes (i.e., chamber enlargement patterns) in only 15 of 169 (10%) cats with HCM, whereas Ferasin and coworkers found morphologic changes in 30 of 61 (50%) cats with HCM. If these data are combined, morphologic changes indicative of chamber enlargement occur in fewer than 20% of cats with HCM. However, this may be an overestimation, because individual animals in these studies might have had more than one morphologic change; we have assumed that each observation is independent, which gives the “best-case scenario.” Thus the sensitivity of ECG in detecting morphologic changes consistent with chamber enlargement or HCM, on the basis of these two studies, is 20%. If one assumes that 15% of the general feline population has heart disease, the positive predictive value of morphologic changes on the ECG is approximately 15%, and the negative predictive value is approximately 85%. Thus a clinician is six times as likely to find a false-positive result as a true-positive result when screening cats by ECG, resulting in substantial expense to clients in pursuit of nonexistent disease. With lower prevalence the probability of a false-positive finding only increases. A negative result would strongly suggest that the cat is “unaffected” because most cats examined are going to be normal. However, most cats with HCM that are examined will also have normal ECG results; these will not be identified.
Presence of pathologic arrhythmias (ventricular premature contraction [VPC], atrial premature contraction [APC], atrial fibrillation) occurred in 17 of 169 (10%) of HCM cats in one study,68 and 8 of 106 (8%) in another study21—again, whether these were independent observations or whether multiple arrhythmias were present in the same cat was not apparent. However, this again results in a sensitivity of 10%, preventing the clinician from effectively ruling out the presence of HCM in the absence of arrhythmias.
Radiography
Most traditional rules of cardiac mensuration (measurement) are of little value in cats. Comparisons of the cardiac silhouette to the thoracic cavity or degree of cardiosternal contact have no value in assessing feline thoracic radiographs for cardiac disease. Sternal contact is prominent in many cats and increases with age in cats with normal hearts.57,59 Similarly, aortic “redundancy” or “undulation,” wherein the ascending aorta forms a prominent silhouette on thoracic films, along with a more sternally positioned heart, is commonly observed in older cats and is an incidental finding.57 One study suggested that this finding is associated with systemic hypertension.60
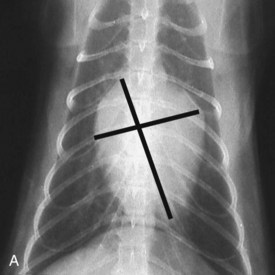
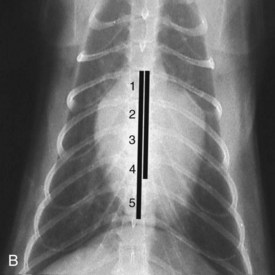
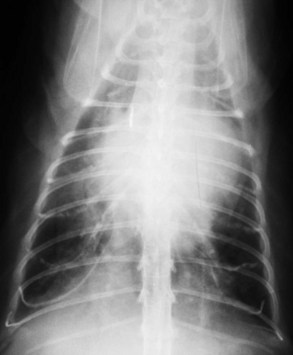
Vertebral heart scale (VHS) has been developed for assessment of cardiac size in cats46 and can help with identification of atrial enlargement or generalized cardiomegaly. A VHS greater than 8.1 is consistent with cardiomegaly in the cat (Figures 20-1 and 20-2). However, clinicians should recognize that the most common adult-onset feline disease (HCM) often does not cause radiographically detectable ventricular enlargement, so a normal VHS does not rule out the presence of significant heart disease in cats.
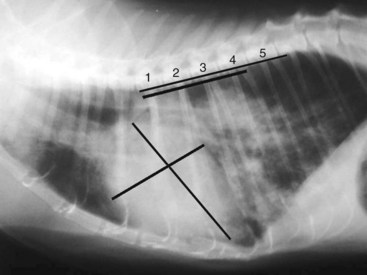
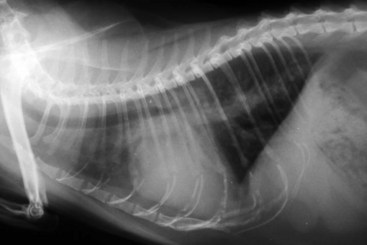
Diagnosis of CHF in cats is aided by thoracic radiographs. Clinicians should not make a diagnosis of CHF in the absence of supportive clinical signs (i.e., radiographs should not be the primary means by which the diagnosis is made). Ideally, marked cardiomegaly is apparent radiographically to support the hypothesis of severe heart disease underlying the pulmonary changes. However, in many cats severe pulmonary changes (pulmonary edema or pleural effusion) obscure the cardiac silhouette, making interpretation of cardiac size impossible. In contrast to dogs, pulmonary edema in cats has little radiographic consistency.50 One study of 23 cats with CHF showed at least six distinct pulmonary parenchymal patterns indicative of pulmonary edema (Figures 20-3 and 20-4).7 Thus pulmonary edema cannot be excluded on the basis of a radiographic pattern that is different from that seen in most dogs. This can complicate the diagnosis of CHF in cats when the cardiac silhouette is not clearly visible.
Biomarkers
NT-proBNP as a Screening Test
Several studies have examined the use of NT-proBNP as a screening test for cardiac disease in cats, namely HCM. While the test showed a difference in NT-proBNP concentrations between “affected” and “unaffected” cats in these studies and good or very good sensitivities and specificities for distinguishing “affected” and “unaffected” cats, most of the studies did not stratify the cats according to severity of subclinical disease. Only two small studies have looked at the ability of NT-proBNP to identify cats with HCM with varying degrees of subclinical disease.35,78 The authors of the first study showed that only cats with severe myocardial hypertrophy (but not moderate or equivocal changes) could be somewhat confidently identified as being “affected.” However, when the authors repeated the study with a new version of the assay, even this ability to detect severely affected cats was compromised. Thus, on the basis of these data, a high NT-proBNP might be expected to help rule in a cat with severe subclinical disease (not many false-positive results) but would not be able to rule out cats with HCM (many false-negative results). Additionally, personal observations by the author suggest that false-positive findings in cats are more common than reported in these studies. Finally, the assay has undergone substantial modifications since these studies were performed and has not yet been re-evaluated. Therefore substantially larger cross-sectional studies are needed, with patients stratified into degrees of subclinical severity and the modified version of the assay used.
NT-proBNP as a Diagnostic Test for Congestive Heart Failure
An alternative use for this assay has been directed at discriminating causes of dyspnea or respiratory distress. Several studies have shown that populations of unaffected cats or cats with dyspnea resulting from acute respiratory disease have lower NT-proBNP concentrations than cats with dyspnea from CHF. However, some overlap exists. One study, by Connolly and coworkers16 showed that approximately 80% of cats would be correctly diagnosed on the basis of NT-proBNP concentrations. However, this also suggests that one in five cats would be incorrectly diagnosed and potentially inappropriately treated, with possible life-threatening consequences.
Feline Hypertension and Heart Disease
Definitions
Systemic hypertension is well documented in cats and can result in significant morbidity and potentially mortality. Clinically, systolic hypertension is identified most commonly, whereas diastolic hypertension has not been reported routinely. Systolic hypertension has been defined as a systolic blood pressure (BP) greater than 160 mm Hg. A grading scheme has been devised, with increasing levels of hypertension presumably associated with worse outcomes (Table 20-1).10
TABLE 20-1 Classification of Systolic Blood Pressure by Risk of Target Organ Damage in the Cat
| Risk Category | Systolic Blood Pressure (mm Hg) | Risk of Target Organ Damage |
|---|---|---|
| I | <150 | Minimal |
| II | 150-159 | Mild |
| III | 160-179 | Moderate |
| IV | >180 | Severe |
Modified from Brown S, Atkins C, Bagley R et al: Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats, J Vet Intern Med 21:542, 2007.
Clinical Signs
Systemic hypertension has been described as a “silent killer” in humans because clinical signs of disease are often inapparent while end-organ damage is occurring. Similarly, in feline patients, few clinical signs are apparent, and they may be subtle and nonspecific, such as anorexia and lethargy. Renal disease can be extremely mild and not clinically apparent. The most common clinical presentation with severe systemic hypertension is acute retinopathy (retinal separation or hemorrhage) (see Figure 29-59).52 Ocular signs may also include dilated pupils and hyphema. Experimentally, acute severe hypertension has resulted in hemorrhagic encephalopathy (“stroke”), but this is not commonly identified in cats with spontaneous disease. Neurologic signs may include head tilt, ataxia, disorientation, and seizures. Cardiac murmurs have been associated with hypertension; however, there is no physiologic reason for systemic hypertension to produce turbulence that would result in a murmur. Thus it is likely that this association is coincidental rather than causal. Several authors have reported cardiac changes in cats with hypertension, including concentric left ventricular hypertrophy and redundancy of the ascending aorta.12,14,34,60 These changes are generally mild but can be confused with a diagnosis of HCM.
Diagnosis
The diagnosis of systemic hypertension in cats is problematic for several reasons. First, the equipment currently available for noninvasive blood pressure (NIBP) measurement is neither accurate nor precise. Despite multiple studies claiming to validate NIBP systems, most have not compared to a true gold standard (direct, invasive telemetric instantaneous BP monitoring). Additionally, many of these studies have examined systems in anesthetized cats rather than conscious animals. Recently, a study comparing oscillometric systems with direct measurements in anesthetized cats found that no system was sufficiently accurate or precise to be useful.2 Recommendations for measuring NIBP in cats have been made by multiple investigators who suggest that four measurements should be made, with the first measurement discarded and the remaining three measurements averaged. However, this method does not guarantee either accuracy or precision of the measurement.
Several NIBP systems are currently marketed and used for measuring systolic blood pressure (SBP) in cats: traditional oscillometric systems; Doppler systems; and, most recently, high-definition oscillometric (HDO) systems. Anecdotal perceptions among clinicians suggested that Doppler-based methodology was more accurate than oscillometric methodology, but unpublished studies comparing both systems in the same animals against invasive measurements have failed to support this perception; both methodologies are equally inaccurate.18b No studies exist detailing performance of HDO systems in cats. However, studies in dogs suggest that these systems would perform no better than standard oscillometric and Doppler systems.87
Complicating the accurate measurement of SBP is the lability of feline BP. The author has observed conscious cats, gently restrained and accustomed to handling, with SBP measurements that vary by as much as 100 mm Hg in the space of a few seconds with little change in heart rate or perceived stress level. Many clinicians attempt to measure SBP at the patient’s home to reduce the impact of stress, but no studies have demonstrated that this strategy results in more accurate measurement. One study examining the effect of conditioning on SBP in dogs showed that repeated measurements over several weeks resulted in a significant gradual reduction in measured BP as the “white-coat” effect subsided in these patients.73 A similar pattern might be anticipated in cats. A study in cats demonstrated a significant white-coat effect in cats that would result in substantially higher SBP measurements than those obtained at rest.6
Clinicians should adopt several steps in the diagnosis of feline systemic hypertension:
1 Examine the appropriate target population. Hypertension is mostly a geriatric disorder in cats. Cats younger than 8 or 9 years of age rarely have systemic hypertension. Therefore BP should not be measured in healthy young to middle-aged cats because the prior probability of these patients actually having hypertension is very low, unless they have renal disease. Indeed, the value of routine BP screening in cats is questionable; it might be more prudent to perform routine urinalysis and an ophthalmic examination and restrict BP measurement to those patients with either inappropriate urine concentrating ability or retinopathy.
2 Obtain multiple measurements over several weeks if an apparently healthy patient is presumptively diagnosed with hypertension on a routine examination. Because hypertension is a chronic progressive disorder, rapid diagnosis is generally not required (unless there is apparent end-organ damage). This can be laborious for both clinician and client, but it reduces the risk of a false-positive diagnosis.
3 Obtain measurements in a quiet environment, using the same technique each time. BP measurement should be performed before the physical examination or any diagnostic procedures, such as blood or urine collection, are performed. Some Doppler machines allow the use of headphones to minimize noise (Figure 20-5). The width of the BP cuff should be 30% to 40% of the circumference of the leg, and it should be positioned at the level of the heart (Figure 20-6). Use the same trained personnel for each measurement to prevent interoperator variability. Record the cuff size and location of the measurement in the medical record along with the BP readings.
4 Accept the lowest reading obtained over several sessions as the most likely.
5 Examine the patient for underlying renal disease (including a urinalysis) and other endocrine disorders that might result in systemic hypertension. If no underlying cause can be identified, reconsider the diagnosis of hypertension.
6 Perform a retinal examination to identify hypertensive retinopathies, especially if the SBP is consistently above 200 mm Hg.
7 Remain skeptical of the diagnosis in every patient in which the diagnosis was unexpected or unexplained.

FIGURE 20-5 Use of headphones with Doppler blood pressure units minimizes noise that might be stressful to the patient.
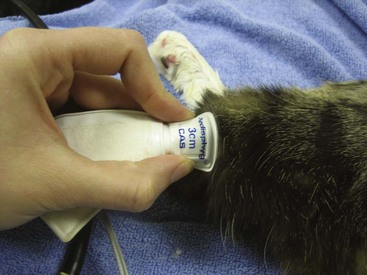
FIGURE 20-6 The width of the blood pressure cuff should be 30% to 40% of the circumference of the leg.
One study recently evaluated the use of NT-proBNP in hypertensive cats with chronic kidney disease (CKD).44 These authors found elevated NT-proBNP concentrations in hypertensive cats with CKD and occasionally in normotensive cats with severe CKD. Thus NT-proBNP might help identify hypertension in cats with CKD in the absence of cardiac disease. Additional larger studies would be necessary to confirm this initial observation.
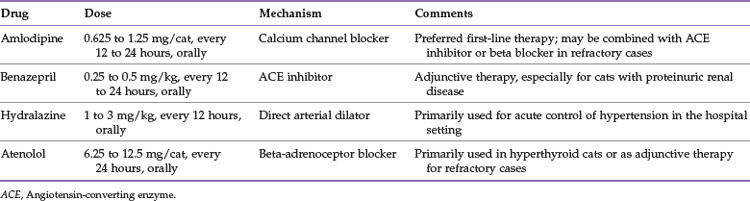
Treatment
Treatment of systemic hypertension generally involves administration of arteriodilators (Table 20-2). The most common drug used for management of feline hypertension is amlodipine. This is usually administered at 0.625 mg per cat, every 12 to 24 hours, by mouth. The medication can be administered transdermally, but reductions in SBP are less predictable and of lesser magnitude than with oral administration.33 Reductions in SBP of 20 to 50 mm Hg can be anticipated with oral amlodipine therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree