Chapter 20 Cardiac Surgery
CLOSED CARDIAC SURGERY
Patent Ductus Arteriosus Ligation
PDA ligation is undertaken through a left fourth thoracotomy in the dog and a left fifth thoracotomy in a cat (Figure 20-1). The most frequent surgical complication is hemorrhage during ductus dissection. If significant hemorrhage occurs during dissection, the ductus should be closed with pledget-buttressed mattress sutures with or without division of the ductus.
Pulmonic and Aortic Valve Dilation
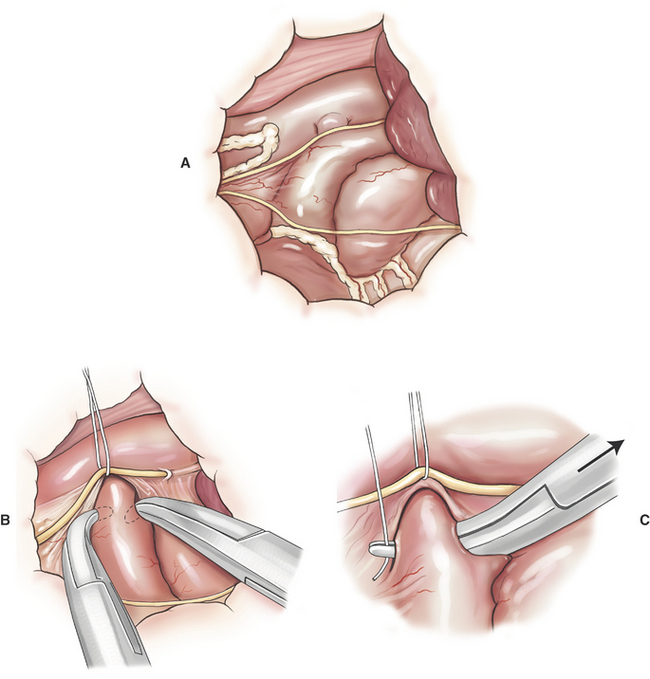
Pulmonic stenosis (PS) and subvalvular aortic stenosis (SAS) are relatively common congenital heart defects in dogs. Despite the relative importance of PS, the natural history of untreated PS in dogs is not well documented. Dogs with moderate PS may tolerate the defect relatively well for many years. Transpulmonic pressure gradients > 100 mm Hg are considered an indication for intervention, especially if animals are exhibiting activity intolerance, syncope, or have concurrent tricuspid regurgitation. The natural history of untreated SAS in dogs is better understood. Dogs with transaortic pressure gradients > 80 mm Hg are known to be at risk for sudden cardiac death early in life. Gradient reduction by valve dilation is assumed, but not proven, to be palliative for dogs with PS. Valve dilation for SAS does not result in sustained decreases in transaortic pressure gradients. Current evidence suggests little or no palliative benefit from valve dilation or surgical treatment of SAS.
Catheter-based balloon valvuloplasty is preferred to surgical valve dilation of PS because it as a less invasive. Surgical valve dilation of PS is indicated for animals that fail balloon-catheter placement across the PS, or when equipment for cardiac catheterization is not available. Surgical valve dilation of PS is performed through a left fourth thoracotomy (Figure 20-2).
Pulmonary Artery Banding
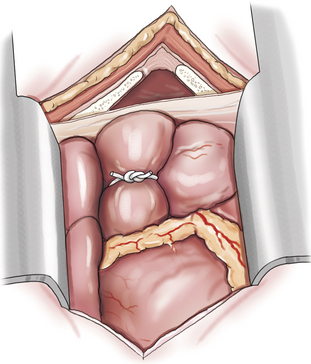
Pulmonary artery banding is performed through a left fourth thoracotomy (Figure 20-3). The appropriate degree of pulmonic constriction is based on pulmonary artery pressure distal to the band and systemic arterial pressures. Pulmonary artery pressure is measured intraoperatively by a catheter introduced through a small purse-string suture in the pulmonary artery. Optimal banding is where pulmonary artery pressure distal to the band is decreased to less than 30 mm Hg (assuming significant pulmonary vascular remodeling is not present), and when the increase in systemic arterial pressure just begins to plateau. As a general rule, optimal banding requires a two-thirds reduction in the diameter of the pulmonary artery although this will vary depending on the degree of pulmonary artery dilation.
Systemic–to–Pulmonary Artery Shunt
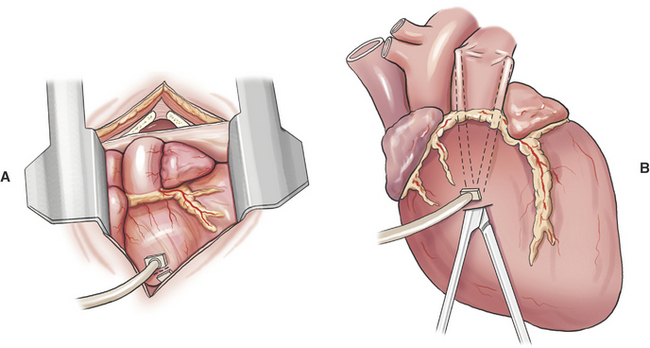
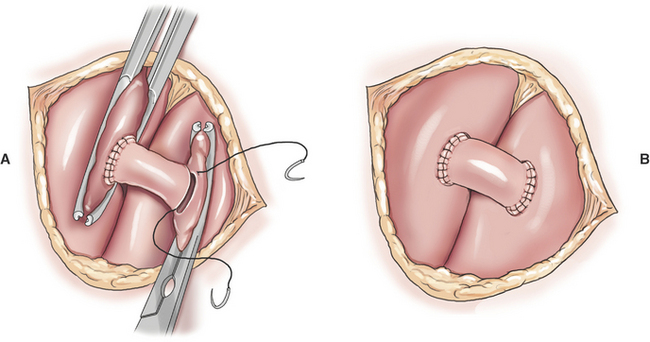
Creation of a systemic–to–pulmonary artery shunt is a palliative surgery for tetralogy of Fallot. The functional goal of a systemic–to–pulmonary artery shunt is to increase pulmonary blood flow without creating an overwhelming left-to-right shunt. The desired result is a measured increase in pulmonary blood flow that lessens hypoxemia by lessening the shunt-to-pulmonary flow ratio. Systemic–to–pulmonary shunt is indicated for animals that have resting cyanosis, debilitating activity intolerance or persistent polycythemia (polycythemia vera > 70%) that requires frequent phlebotomy. Most veterinary experience is based on various modifications of the classic Blalock-Taussig shunt. The original Blalock-Taussig shunt consisted of dividing the left subclavian artery and performing an end-to-side anastomosis of the distal end of the divided artery to the pulmonary artery. In animals, the left subclavian artery generally does not have sufficient length to reach the pulmonary artery without kinking. Several modifications of the classic procedure have been devised including a synthetic vascular graft matched in size to the subclavian artery, harvesting the left subclavian artery as a free autogenous graft, or using autogenous jugular vein. Animals can receive significant palliation from any of the previous methods so long as pulmonary blood flow is increased to an appropriate degree.
A modified Blalock-Taussig shunt is performed through a left fourth thoracotomy (Figure 20-4). A continuous thrill should be palpable on the pulmonary artery and hypoxemia should be lessened immediately after surgery.
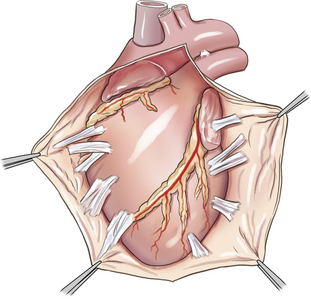
Pericardiectomy
Pericardiectomy can be performed via either a right or left thoracotomy, or a median sternotomy. Median sternotomy has the advantages of providing access to both ventricles and requiring less cardiac manipulation, and thus is preferred by many surgeons (Figure 20-5). Excision of the pericardium ventral to the phrenic nerves (i.e., subphrenic pericardiectomy) is adequate in most cases. In animals with constrictive pericarditis, the pericardium may have to be separated from the epicardium by blunt and sharp dissection. Additionally, epicardial decortication may be necessary to relieve constrictive physiology in animals with pericarditis. Epicardial decortication entails careful separation of a fibrous layer from the myocardium by sharp dissection. Decortication should not be attempted over the atria or portions of the ventricles containing major coronary vessels.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree