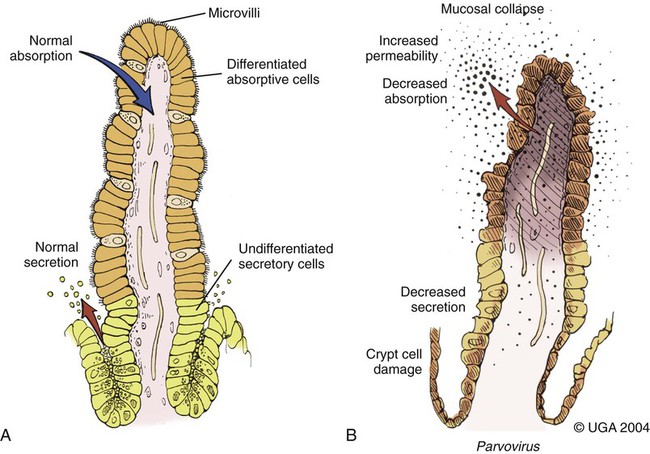
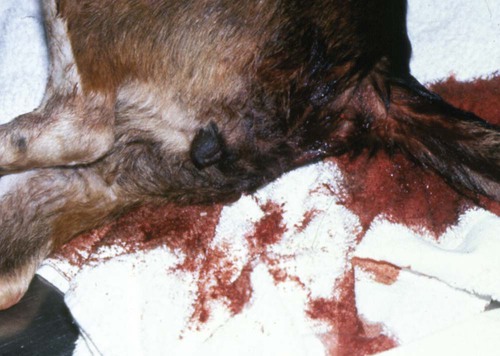
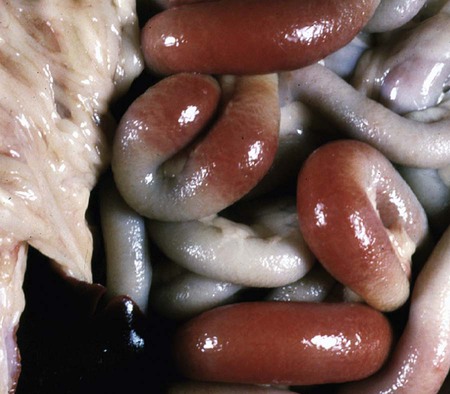
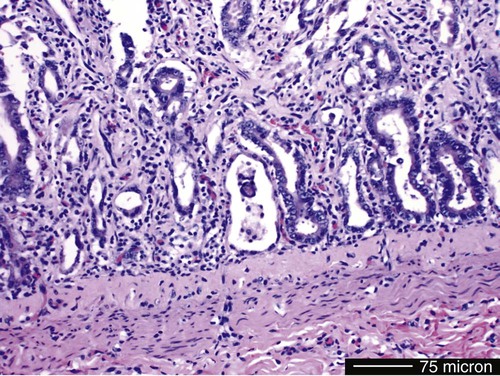
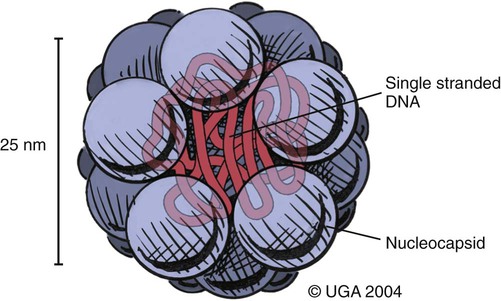
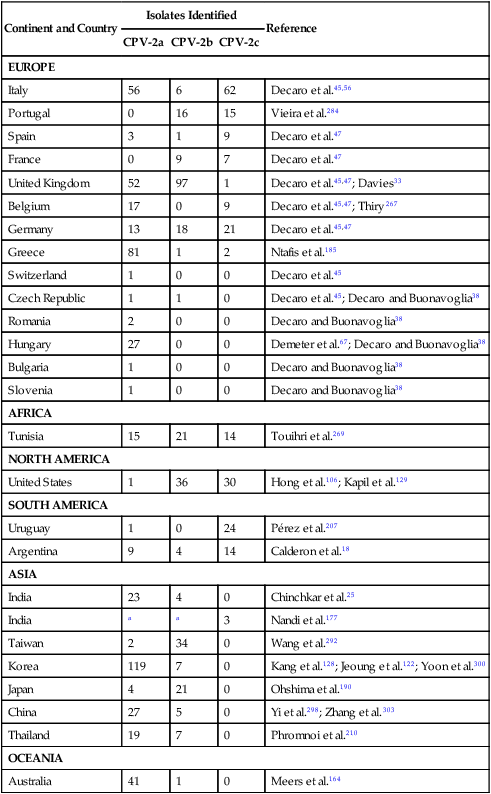
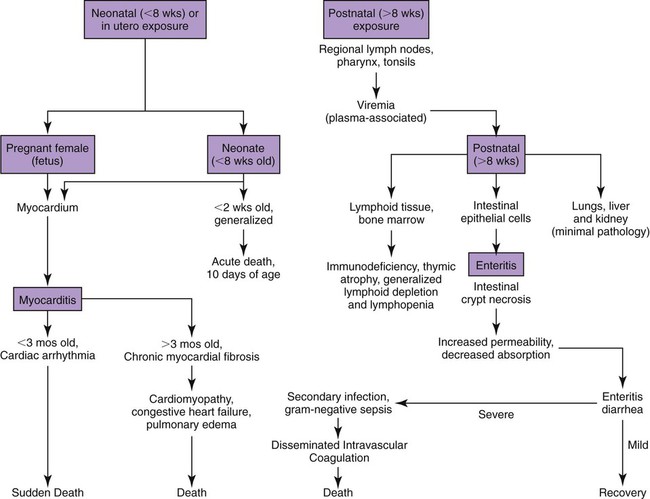
Viral enteritis is one of the most common causes of infectious diarrhea in dogs younger than 6 months of age. Canine parvovirus (CPV)-2 and canine coronavirus (CCoV) have been incriminated as primary pathogens. CPV-1 and canine rotaviruses (CRV) can produce mild to inapparent illness in young pups (less than 8 weeks old), and their clinical significance is considered low. Astrovirus, herpesvirus, enteroviruses, calicivirus, parainfluenza virus, reovirus, and other virus-like particles have been isolated from or identified in feces from dogs with diarrhea, but their pathogenicity is uncertain.* CPVs are small, nonenveloped, DNA-containing viruses that require rapidly dividing cells for replication (Fig. 8-1). As is the case with all parvoviruses, CPV-2 and -1 are extremely stable and are resistant to adverse environmental influences (see Husbandry). For the following discussion, the term CPV will be used as a general term for canine parvovirus strains; specific strains will be designated by number. Canine parvovirus enteritis is probably one of the most common infectious disorders of dogs and the most prevalent virus in dogs with infectious diarrhea.287,297 This highly contagious, often fatal, disease is caused by strains of CPV-2 (2, 2a, 2b, and 2c). The virus, along with other carnivore parvoviruses, belongs to the feline parvovirus subgroup within the genus Parvovirus.272 CPV-2 evolved from an unknown parvovirus source over a 10-year period before its complete adaptation to dogs and pandemic spread in the late 1970s.19,206,206 Subsequently, as a result of mutations and immune selection, the original CPV-2 strain has undergone genetic mutations in the dog, with development of new strains of the virus.201,202,202 In 1980, the original strain of CPV-2 evolved into type 2a (CPV-2a); in 1984, another variant designated type 2b (CPV-2b) appeared. In 2000, another strain (CPV-2c) was originally isolated in dogs in Italy.15,151 This strain, known as Glu426, has a substitution of the amino acid at the 426 position from asparagine/aspartic acid to glutamic acid and altered the viral capsid antigenic site, epitope A. It has rapidly spread and is one of the major isolates worldwide.* These CPV alterations were associated with a genetic adaptation and change in the capsid epitope B region, enabling the parvovirus to replicate and spread more effectively in susceptible dogs, in addition to the ability to infect cats. Multiple strains have also been identified co-infecting an individual dog or cat.8,105,105 Furthermore, there is initial evidence for recombination between feline panleukopenia virus (FPV) and CPV strains in nature.190 The relative numbers of each strain of CPV that have been reported in various countries of the world are summarized in Web Table 8-1. CPV-2 strains are infrequently reported and the isolates are of the 2a, 2b, or 2c strains. In South America, Europe, and northern Africa, all three have been observed in many countries where large numbers of samples have been obtained. The 2b and 2c isolates predominate in North America. In Asia and in isolated island nations with importation restrictions, such as the United Kingdom, Japan, and Australia, the 2a and 2b strains predominate.33,45,45 WEB TABLE 8-1 Numbers of Each Strain of CPV Reported in Various Countries aSixteen samples were positive for a or b; which one was not determined. Genetic mutations in the structure of the surface transferrin receptor of the virus have resulted in structural alterations that control the host adaptation of CPV strains and may influence cross-reactivity in immunologic testing.97,113,113 In side-by-side comparisons, genetically different strains may vary in their virulence.176 The clinical relevance of these structural changes and the degree of heterologous cross-protection between strains need further clarification (see Heterologous Strain Protection, under Prevention). For a further discussion of CPV strains in cats, see Canine Parvovirus Infection of Cats in Chapter 9. Natural CPV infections have been reported in domestic dogs (Canis familiaris), bush dogs (Speothos venaticus), coyotes (Canis latrans), wolves (Canis lupus), crab-eating foxes (Cerdocyon thous), and maned wolves (Chrysocyon brachyurus); most if not all Canidae are susceptible. Experimental infections can be produced in domestic ferrets, mink, and cats; however, the infection is generally self-limiting. A related, but genetically distinct, parvovirus has been isolated from raccoons.130 The original CPV-2 isolates produced only systemic and intestinal infections in dogs,274 whereas the newer type 2a and 2b strains may infect felines under experimental167,173,173 and natural171,273 circumstances (see Feline Parvovirus Infection, Chapter 9). In domestic dogs, CPV infection does not necessarily result in apparent disease; many dogs that become naturally infected never develop overt clinical signs, especially in the presence of residual maternally derived antibody (MDA). When the disease occurs, clinical illness is most severe in young, rapidly growing pups that harbor intestinal helminths, protozoa, and certain enteric bacteria such as Clostridium perfringens, Campylobacter spp., and Salmonella spp. In susceptible animals, the incidence of severe disease and death can be very high. CPV is highly contagious, and most infections occur as a result of contact with contaminated feces in the environment. In addition, humans, instruments (equipment in veterinary facilities or grooming operations), insects, and rodents can serve as vectors. Dogs may carry the virus on their haircoat for extended periods. The incubation period of the original CPV-2 strains in the field was 7 to 14 days; experimentally, the incubation period was 4 to 5 days. With CPV-2a, -2b and -2c strains, the incubation period in the field can be as brief as 4 to 6 days.40,48,48 Acute CPV enteritis can be seen in dogs of any breed, age, or sex. Nevertheless, pups between 6 weeks and 6 months of age and Rottweilers, Doberman pinschers, Labrador retrievers, American Staffordshire terriers, German shepherds, and Alaskan sled dogs seem to have an increased risk.90,101,101 Some outbreaks of severe gastroenteritis and mortality due to CPV-2c infections in adult dogs (more than 6 months old) have been reported.18,43,43 CPV spreads rapidly from dog to dog via oronasal exposure to contaminated feces (Web Fig. 8-1). Virus replication begins in lymphoid tissue of the oropharynx, mesenteric lymph nodes, and thymus and is disseminated to the intestinal crypts of the small intestine by means of viremia. Marked plasma viremia is observed 1 to 5 days after infection. Subsequent to the viremia, CPV localizes predominantly in the gastrointestinal (GI) epithelium lining the tongue, oral and esophageal mucosae, and small intestine and lymphoid tissue, such as thymus, lymph nodes, and bone marrow. It can also be isolated from the lungs, spleen, liver, kidney, and myocardium.294 Normally, intestinal crypt epithelial cells mature in the small intestine and then migrate from the germinal epithelium of the intestinal crypts to the tips of the villi (Fig. 8-2, A). After reaching the villous tips, the intestinal epithelial cells acquire their absorptive capability and aid in assimilating nutrients. Parvovirus infects the germinal epithelium of the intestinal crypts, causing destruction and collapse of the epithelium (see Fig. 8-2, B). As a result, normal cell turnover (usually between 1 and 3 days in the small intestine) is impaired, and the villi become shortened. CPV also destroys mitotically active precursors of circulating leukocytes and lymphoid cells. In severe infections, the results are often neutropenia and lymphopenia. Secondary bacterial infections from gram-negative and anaerobic microflora cause additional complications related to intestinal damage, bacteremia and endotoxemia, and disseminated intravascular coagulation.192,276,276 Active excretion of CPV-2 strains begins on the third or fourth day after exposure, generally before overt clinical signs appear. CPV-2 is shed extensively in the feces for a maximum of 7 to 10 days postinoculation as determined by virus isolation and enzyme-linked immunosorbent assay (ELISA) methods. However, by using nucleic-acid detection assays (real-time polymerase chain reaction [PCR]), the CPV-2 variants (a, b, c) have been detected in the feces for several weeks after infection.40,48 Development of local intestinal antibody is most likely important in the termination of fecal excretion of parvovirus. Serum antibody titers can be detected as early as 3 to 4 days after infection and may remain fairly constant for at least 1 year. CPV enteritis may progress rapidly, especially with the newer strains (a, b, c) of CPV-2. Vomiting is often severe and is followed by diarrhea, anorexia, and rapid onset of dehydration. The feces appear yellow-gray and are streaked or darkened by blood (Fig. 8-3). Elevated rectal temperature (40° to 41° C [104° to 105° F]) and leukopenia (mainly lymphopenia) may be present, especially in severe cases. Total leukocyte counts may be even within reference ranges because of concomitant virus-induced lymphopenia and neutrophilia consequent to infections by opportunistic bacteria. Those developing systemic inflammatory response syndrome have a greater risk of mortality.125a Death can occur as early as 2 days after the onset of illness and is often associated with gram-negative sepsis or disseminated intravascular coagulation, or both. Primary neurologic disease may be caused by CPV but more commonly occurs as a result of hemorrhage into the central nervous system (CNS) from disseminated intravascular coagulation or from hypoglycemia during the disease process, sepsis, or acid-base-electrolyte disturbances.2 Concurrent infection with viruses such as canine distemper virus is also possible. Grossly visible cerebellar hypoplasia, common in kittens prenatally or neonatally infected with FPV, has not been frequently associated with CPV infection. CPV DNA was amplified using PCR from brain tissue of two dogs with cerebellar hypoplasia, but time of exposure to CPV was not mentioned.241 CPV was detected by immunohistochemistry and in situ hybridization in CNS lesions of inbred pups with a leukoencephalopathy.241a CPV was identified by immunohistochemistry in many cell types (including neurons) within the CNS of pups with generalized tremors and dysmetric pelvic limb gait.248 Mild to moderate lymphohistiocytic meningitis or leukoencephalitis was found and, in one case, there was cerebellar and cerebral white matter vacuolation. Immunohistochemistry failed to identify CPV-2 antigens in brains of dogs279 with signs of parvovirus enteritis and no clinical CNS manifestations but with mild histopathologic neurodegenerative changes. However, CPV DNA60 and messenger RNA (mRNA)76 were detected even at high titers in the brains of dogs without neurologic signs, indicating active replication of the virus in the nervous tissues. In addition, CPV-2 sequences have been detected in cat brains by the same investigators; however, PCR-negative controls were not included to eliminate the possibility of laboratory contamination of the processed samples and further investigations are warranted (see also Central Nervous System Infection under Feline Parvovirus Infection, Chapter 9). Erythema multiforme was diagnosed in a dog with parvovirus enteritis.81 Skin lesions included ulceration of the footpads, pressure points, and mouth and vaginal mucosa. Vesicles in the oral cavity and erythematous patches on the abdomen and perivulvar skin were also present. Parvovirus was confirmed in the affected cells by immunohistochemistry. CPV myocarditis can develop from infection in utero or in pups younger than 6 weeks of age. All pups in a litter are usually affected. Pups with CPV myocarditis often die, or they succumb after a short episode of dyspnea, crying, and retching. Signs of cardiac dysfunction can be preceded by the enteric form of the disease or may occur suddenly, without apparent previous illness. The spectrum of myocardial disease in individuals is wide and can include any of the following: acute diarrhea and death, without cardiac signs; diarrhea and apparent recovery followed by death, which occurs weeks or months later as a result of congestive heart failure; or sudden onset of congestive heart failure, which occurs in apparently normal pups at 6 weeks to 6 months of age. Myocardial disease has become progressively less common in parvovirus-infected dogs since the original pandemic spread of CPV-2 in the late 1970s.2 Subsequent to this outbreak, the majority of bitches have been vaccinated or exposed to CPV strains and developed strong humoral immune responses; therefore, the high titer of MDA in nursing pups prevents neonatal infection with virus in the early period of life when myocardial cell replication is occurring. Myocarditis is still occasionally found in pups that do not nurse sufficiently or are born to isolated, unvaccinated bitches.288 Myocarditis, with or without enteritis, has been associated with natural CPV-2a and -2b infections in 6- to 14-week-old dogs from Korea.295 CPV infection appears not to be a common cause of heart disease because PCR analysis at necropsy of 27 dogs with either dilated cardiomyopathy or myocarditis did not detect CPV in any of the samples.159 Asymptomatic urinary tract infection has been detected in approximately 25% of pups after CPV enteritis.131 This predisposition was attributed to fecal contamination of the external genitalia in association with neutropenia. Untreated subclinical urinary tract infection may lead to chronic urinary infection as an undesirable consequence. Bacteria from GI or environmental origin have been isolated from the intravenous catheters removed from dogs being treated for suspected parvovirus infections.141 Most of these organisms were gram-negative types (Serratia, Acinetobacter, Citrobacter, Klebsiella, and Escherichia). Most organisms were resistant to penicillins, first-generation cephalosporins, and macrolides while being susceptible to aminoglycosides, quinolones, chloramphenicol, potentiated sulfonamides, and clavulanate-potentiated penicillins. Despite the positive culture results of the catheter tips, none of the dogs showed systemic clinical signs of infection, and only one developed local phlebitis. The sudden onset of foul-smelling, bloody diarrhea in a young dog (under 2 years of age) is often considered indicative of CPV infection. However, not all dogs with bloody diarrhea (with or without vomiting) are necessarily infected with CPV, and nonhemorrhagic diarrhea is often caused by CPV. Parasitic or enteropathogenic bacterial infections, alone or in combination, should also be considered64 (see Chapter 37), as well as other viral infections, including enteric and pantropic CCoVs.37 All clinical signs characteristic of CPV infection are seldom present at any one time. Leukopenia, although not found in all dogs, is usually proportional to the severity of illness and the stage of disease at the time the blood is taken. Monitoring of leukocyte changes may yield prognostic information about the likely course of infection.91 Pups dying from the disease generally have total leukocytes equal to or less than 1030 cells/µL and have persistent lymphocytopenia, monocytopenia, and eosinopenia within the first 3 days of hospitalization. Abnormal coagulation test results may include prolongation of the activated partial thromboplastin time, increased thromboelastogram amplitude, and decreased antithrombin III activity.194 In dogs with parvovirus enteritis, serum total cholesterol and high-density lipoprotein cholesterol levels decreased and triglyceride levels were increased, whereas increases in the cholesterol levels were proportional to the severity of illness.299 Similarly, high serum cortisol and low serum thyroxine concentrations 24 and 48 hours after hospitalization were associated with mortality in dogs with parvovirus diarrhea.243 Cardiac troponin I is a plasma marker for myocardial damage. It has been used to detect cardiac damage after traumatic or infectious diseases in dogs. Dogs of 6 months to 4 years of age, suffering from parvovirus infection, did not have elevated levels.73 This suggests the myocardial damage is restricted to very young puppies. Erythrocyte oxidative stress indices were significantly higher in dogs with gastroenteritis and CPV-positive fecal PCR results than in those with negative results.196 Fecal ELISA antigen tests are available for in-hospital testing for CPV infection (see Web Appendix 6). These tests are specific but poorly sensitive for detecting CPV infection.* Using ELISA, the period of fecal virus shedding is usually brief, corresponding to the first few days of clinical illness. With an incubation period ranging from 4 to 6 days, CPV strains are seldom detectable by ELISA longer than 10 to 12 days after natural infection, and shedding can be intermittent. Therefore, negative results during or after this time period do not eliminate the possibility of CPV infection. False-negative results obtained with the ELISA-based methods have been associated to early appearance of antibodies that may sequester CPV particles in the intestinal lumen, thus preventing subsequent binding to the monoclonal antibody used in the test.69 There is no significant difference in the ability of the ELISA tests to detect the CPV-2 variants, and the recently emerged CPV-2c is detected at same rates as CPV-2a or -2b.46 Negative results on CPV-ELISA testing could be confirmed by PCR methods. Positive results confirm infection or may be found with some of the commercial assay methods after administration of attenuated live CPV vaccines. In contrast to the usual strong positive results after natural infection, vaccine virus may yield a weak false-positive result in dogs 4 to 8 days after vaccination. A slide agglutination test, using porcine erythrocytes, has also been developed to detect CPV-2 in fecal and intestinal samples.157 Hemagglutination using porcine or feline erythrocytes can be used for CPV-2 detection, but it has been proven to be only slightly more sensitive than ELISA and poorly specific for the possible presence of isoagglutinins in fecal samples or other hemagglutinating viruses (mainly reoviruses). Moreover, this method requires the constant availability of erythrocyte donors.69 Other immunoassay procedures have been used to detect presence of virus in feces or tissues (see Pathologic Findings). CPV typically produces lesions in the jejunum, ileum, mesenteric lymph nodes, and other lymphoid tissues. CPV can be isolated from these tissues or feces using tissue culture systems, if performed early. Later in the course of disease, virions become coated by antibodies and cleared. In most tissues, intranuclear inclusions are observed. In the glossal epithelium, these may appear as being within the cytoplasm, when in fact they originate in the nuclear space.115 Immunochemical methods can also be used, in conjunction with light or electron microscopy (EM), to detect virus in tissue culture, feces or tissues (see Pathologic Findings). Nucleic acid amplification methods based on viral DNA have greatly increased the sensitivity of viral detection.69 Not only has PCR methodology been a sensitive means of detecting CPV in feces of infected dogs,26,167,173,281 but quantitative assays (real-time PCR) can also provide an estimation of the viral DNA load.52,133a Whereas immunochemical and virus isolation methods generally detect virus in feces for less than 10 days of infection, quantitative PCR results for CPV-2c have peaked at 10 days and have remained positive, albeit at lower levels, for as long as 54 days.48 Whether PCR-detected virus is infectious remains to be determined by transmission studies. PCR also detects vaccine virus in blood and feces for at least 2 weeks after vaccination.244a,285 Quantitative virus was able to distinguish between virus from vaccination versus natural infection by having a higher viral load in the latter instance.285 In addition, real-time PCR assays using minor groove binder probes are now available for specific identification of CPV-2 variants circulating in the field53 and for discrimination between field and vaccinal strains.51,58 These assays are particularly useful to rule out potential reversion to virulence of the vaccine virus or, more frequently, the simultaneous detection of vaccine and field CPV strains in pups displaying acute gastroenteritis shortly after CPV vaccination.49 Serology is not the best method to diagnose CPV infection, because most dogs are vaccinated against it or have been previously exposed to the virus. In contrast, serologic tests can be usefully employed to evaluate the MDA titers in pups to be vaccinated. As a general rule, parvoviruses cause hemagglutination of erythrocytes. Inhibition of porcine erythrocyte hemagglutination by CPV, by adding test sera can be used to demonstrate the presence of CPV specific serum antibody.157 The presence of high hemagglutination inhibition (HI) titer in a single serum sample collected after a previously unvaccinated dog has been clinically ill for 3 or more days is diagnostic for CPV infection. Rising titers (seroconversion) can also be demonstrated when acute and 10- to 14-day convalescent serum samples are compared using either canine or feline parvovirus in HI and virus neutralization (VN) tests. ELISA tests are also available that permit distinction between IgG and IgM.235 In-office ELISA test kits are commercially available for semiquantitative IgG and IgM measurements (Immunocomb, Biogal Labs, Megiddo, Israel)289–291 and for determining adequate IgG titers for vaccination (TiterCHEK CPV/CDV Test Kit, Synbiotics, San Diego, CA). See Web Appendix 6 for further information on these products. Early lesions are most pronounced in the distal duodenum; later, the jejunum is more severely affected. The intestinal wall is generally thickened and segmentally discolored, with roughening or fibrin adhering on the serosal surfaces; within the lumen there is denudation of intestinal mucosa and the presence of dark, sometimes bloody, watery material within the stomach and intestine (Fig. 8-4). In mild cases, the lesions are not easy to distinguish from those of nonspecific enteritis. Enlargement and edema of thoracic or abdominal lymph nodes have been observed. The intestinal lesions are characterized by necrosis of the crypt epithelium in the small intestine.261 Intranuclear viral inclusion bodies may be seen in these epithelial cells and throughout the squamous epithelia of the upper GI tract.158 The pathologic changes may range from mild inflammation to diffuse hemorrhagic enteritis. The villi are shortened or obliterated, owing to lack of epithelial replacement by maturing crypt cells, resulting in collapse of the lamina propria (Fig. 8-5). Necrosis and depletion of the lymphoid tissue (e.g., Peyer’s patches; mesenteric lymph nodes, thymus, and spleen) are present. Pulmonary edema or alveolitis may be observed in dogs dying of complicating septicemia.277 Histologic examination is usually definitive; however, specific identification of parvovirus in tissue specimens can be done by immunofluorescence or other immunochemical methods. Using indirect fluorescent antibody testing, antigen in dogs with lethal CPV enteritis can be found in the dorsal side of the tongue (96.3%), pharynx (81%), esophagus (50%), ventral tongue (20.4%), planum nasale (5.6%), small intestinal mucosa (85.2%), bone marrow (81.6%), spleen (79.6%), thymus (66.7%), mesenteric nodes (50.4%), palatine tonsils (58.5%), and myocardium (1.9%).294 In situ hybridization or quantitative PCR methods have been valuable specific tools for virus identification in tissue specimens.76,287 Virus can be detected with greater prevalence in tongue as compared to other tissues when there is autolysis or freezing and thawing before necropsy.162 Quantitative PCR of naturally infected dogs has shown similar tissue distribution among viral strains with highest concentrations of virus in lymphoid tissues, intermediate levels in nervous tissue, and lowest levels in urinary bladder.59 Parvovirus myocarditis, when present, is recognized grossly as pale streaks in the myocardium (Fig. 8-6). The myocardial lesions consist of a nonsuppurative myocarditis with multifocal infiltration of lymphocytes and plasma cells within the myocardium. Basophilic intranuclear inclusion bodies have been observed in cardiac muscle fibers, and parvo-like virus particles have been demonstrated by EM and by in situ hybridization288 in the inclusion bodies. The primary goals of symptomatic treatment for CPV enteritis are restoration of fluid and electrolyte balance and preventing secondary bacterial infections. Antimicrobial agents, motility modifiers, and antiemetic agents are given in Table 8-1. Fluid therapy is probably the single most important aspect of clinical management and should be continued for as long as vomiting or diarrhea (or both) persists. Hypoglycemia and hypokalemia are common and should be corrected through additions to the IV fluids. Antimicrobial agents are recommended because the combination of severe disruption of the intestinal epithelium allowing bacteria into the blood and peripheral neutropenia increases the risk of sepsis.232 The most common bacteria appear to be Escherichia coli and C. perfringens.276,277 The best antibacterial spectrum (for gram-negative aerobic and anaerobic bacteria) is provided by combination of a penicillin and an aminoglycoside. Before a nephrotoxic drug such as an aminoglycoside is administered, the patient should be fully hydrated. Should nephrotoxicity of aminoglycosides be a concern, parenteral third-generation penicillins or cephalosporins can be used as sole treatment alternatives to achieve the desired spectrum. Quinolones, which have a good gram-negative aerobic antibacterial spectrum, must be avoided in young growing dogs. Antiemetic drugs are helpful to reduce fluid loss and decrease patient distress and allow for enteral nutrition. Metoclopramide hydrochloride and prochlorperazine have proved helpful in most dogs with persistent vomiting. The serotonin receptor antagonists have been recommended as the most efficacious antiemetics.145,189 Ondansetron and dolasetron have both been used in dogs. The use of antiemetics in this disease is controversial as they do not always curtail the vomiting; furthermore, they can lead to hypotension.149 Drug therapy to alter gut motility is seldom recommended in the treatment of CPV enteritis. If needed, narcotic antispasmodics (e.g., diphenoxylate hydrochloride, loperamide hydrochloride) are preferred when motility modifiers are needed. TABLE 8-1 Drug Therapy for Canine Viral Enteritis IM, Intramuscular; IV, intravenous; PO, by mouth; prn, as needed; SC, subcutaneous. aDose per administration at specified interval. For additional information on these drugs, see the Drug Formulary in the Appendix. bSlow infusion can be used for severe vomiting. cAdministered after correction of dehydration. eSEPTI-serum; Immvac Inc., Columbia, MO. (Based on a concentration of >320 mg of IgG/mL.) Although withholding food and water are general recommendations in treating GI diseases, including parvovirus enteritis, information suggests this is not necessary. When dogs with parvoviral enteritis were fed, beginning on the first day of treatment (via nasoesophageal tube), their recovery time was shortened, and they maintained body weight when compared with dogs that were treated the conventional method of withholding food until signs had ceased for 12 hours.175 During the initial stage of CPV enteritis, recommended adjunctive therapy has included transfusion of specific hyperimmune plasma or administration of antiendotoxin sera72 (see Passive Immunization, Chapter 100, and the Drug Formulary in the Appendix). These adjuncts reportedly decrease mortality and the length of hospitalization72 but are expensive. A recombinant bactericidal-permeability-increasing protein, which counteracts endotoxin, did not alter clinical outcome or survival in dogs naturally infected with CPV.193 This result is despite increases in plasma endotoxin in affected animals. Recombinant human granulocyte colony-stimulating factor (G-CSF) has been advocated for the treatment of severe neutropenias induced by CPV infection.84 However, supplementing recombinant human G-CSF to neutropenic pups with CPV infection did not change any aspect of their clinical outcome.166,233,233 The lack of efficacy of exogenous G-CSF was thought to be the result of already existent high levels of endogenous G-CSF that are maximally stimulating the production of neutrophils.27 However, in a controlled study using recombinant canine G-CSF, treated dogs had shorter durations of hospitalization and higher neutrophil counts; however, survival times were reduced.73a Therefore, use of G-CSF is not recommended until additional studies are performed. Use of the influenza virus neuraminidase inhibitor oseltamivir phosphate (Tamiflu, Roche) has been recommended for treating parvovirus-infected dogs. There is no theoretical or actual benefit to its use with parvovirus infection. In one controlled study involving naturally infected dogs, those given placebo versus oseltamivir had significantly greater weight loss and leukopenia; however, there was no other difference in clinical outcome.239a There has been theoretical speculation that the drug may act somehow on intestinal bacteria; however, this is not substantiated. See the Drug Formulary in the Appendix. Dogs with experimental and natural parvovirus infection have been treated with recombinant feline interferon, IFN-ω, in high intravenous (IV) dosages (2.5 × 106 units/kg) beginning early (4 days or less after infection) in the course of parvovirus infection.66,119,133,155,165 Reduced signs of clinical illness and mortality were observed in treated dogs. See the Drug Formulary in the Appendix, for further information on its availability and use. Several therapies have been recommended and would seem of empirical benefit, but they have not been examined well enough to indicate that they are efficacious.144 Some puppies are severely anemic, which may be the result of GI loss of blood caused by the parvovirus enteritis, or it might be unrelated to parvovirus, such as blood loss related to parasitism. Transfusion of whole blood might benefit these puppies. Hypoproteinemia is present in some puppies. A whole blood transfusion will help resolve the problem, but if erythrocytes are not needed, a more appropriate therapy is plasma transfusion. Plasma can provide both immune globulins (see later discussion) and colloidal albumin. Ideally, serum albumin concentration should be maintained at 2.0 g/dL or greater. If edema is present as a result of decreased proteins and is not corrected by a plasma transfusion, then synthetic colloid such as hetastarch should be considered. Colloids should not be given until dehydration is corrected. Glucocorticoids and flunixin meglumine may have beneficial effects in treating early sepsis or endotoxemia. These agents should not be used until dehydration is corrected, and repeated doses should not be given. The use of hyperimmune plasma might be questioned because, at the time of clinical signs, the colonization of the target organs is completed and the levels of antibodies may be increased. However, pups that had a delayed or lower serum antibody response are often more severely affected. Canine lyophilized IgG has been beneficial in treatment of dogs with naturally occurring CPV infection.146 Compared with control dogs, those receiving IgG as adjunctive therapy had reduced severity of disease, reduced cost of treatment, and reduced hospitalization time. Similarly, experimentally infected dogs receiving immunoglobulins against CPV derived from chicken egg yolk were protected from clinical illness when sufficient quantities were given.282 Because commercial immunoglobulin products are not available in all countries, plasma or blood transfusions from dogs with high levels of antibodies to CPV may be the most practical means of providing immediate protection against viremia. A puppy that recovers from CPV enteritis is immune to reinfection for at least 20 months and possibly for life. On reexposure to the various strains of CPV-2, protected pups will not have increased serologic titers, show overt signs of illness, or shed virus in the feces. In general, a good correlation exists between serum antibody titer, determined by either HI or VN testing, and resistance to infection. Serum antibody titers remain high for a prolonged period after CPV enteritis, even if reexposure does not occur. If serum antibody titers become low, a localized infection is possible, but viremia and generalized illness are unlikely to develop. Although it may help in protection against entry of CPV, intestinal secretory IgA probably does not play a role in the longevity of protective immunity because intestinally derived antibody titers do not persist for longer than 15 days after infection.212 In addition to the information provided in this section, Chapter 100 should be consulted regarding vaccination for this disease. Commercially prepared inactivated CPV vaccines have generally been replaced by attenuated vaccines that provide superior immunity. Attenuated vaccines are safe either alone or in combination with other vaccine components. In the absence of maternal antibody blockade, the onset of protection against CPV infections is as early as 3 days postvaccination. Transient lymphopenia may occur 4 to 6 days after the primary administration of some attenuated CPV vaccines. Most attenuated live CPV vaccine strains replicate in the intestinal tract and are briefly shed in the feces. Although concern has been expressed about the possibility of attenuated CPV vaccine undergoing reversion of virulence and causing apparent disease, experimental studies have shown that these attenuated live virus CPV vaccines are safe.125 The events after administration of attenuated live CPV vaccines parallel those after wild-type CPV infection. On day 2 after subcutaneous administration of vaccine, viremia and systemic distribution occur, with shedding from the GI tract on days 3 to 8. One difference between vaccine-induced and wild-type infections is that lower quantities of virus are shed after vaccination. Humoral immune responses to attenuated live vaccines are similar to those observed with wild-type infection. Serum antibody is usually detectable 3 days after vaccination, with levels rising rapidly to those observed after subsequent natural infection. Even if reexposure does not occur, protective antibody titers may persist for at least 2 years, and dogs exposed during this time should not become infected. Vaccination with potentiated (high-titer) attenuated CPV vaccine has been shown to protect dogs on subsequent experimental challenge exposure.245 On the basis of serum antibody titers, in a veterinary hospital setting, 27% of the dogs being evaluated for revaccination had titers below the protective level for CPV.161 Although serum antibody titers are not absolute indicators of protection, positive results have a good correlation with protection against CPV infection (see also Canine Parvovirus Enteritis Chapter 100). Even systemic chemotherapy for neoplasia in dogs did not affect serum CPV-2 antibody titers.103 Antibody titer comparisons may be misleading because many dogs with low titers that have been previously boostered against CPV are protected with subsequent challenge. These challenge experiments are considered to be the gold standard for protection. A number of challenge studies have been conducted by major vaccine companies demonstrating a minimum of 3 years duration of immunity for attenuated live canine parvovirus in combination vaccines.1,89,89 Other studies have shown a duration of immunity of at least 7 years for multivalent vaccines containing CPV.244 These results will be available in future product literature and labeling.
Canine Viral Enteritis
Canine Parvovirus Enteritis
Etiology
Continent and Country
Isolates Identified
Reference
CPV-2a
CPV-2b
CPV-2c
EUROPE
Italy
56
6
62
Decaro et al.45,56
Portugal
0
16
15
Vieira et al.284
Spain
3
1
9
Decaro et al.47
France
0
9
7
Decaro et al.47
United Kingdom
52
97
1
Decaro et al.45,47; Davies33
Belgium
17
0
9
Decaro et al.45,47; Thiry267
Germany
13
18
21
Decaro et al.45,47
Greece
81
1
2
Ntafis et al.185
Switzerland
1
0
0
Decaro et al.45
Czech Republic
1
1
0
Decaro et al.45; Decaro and Buonavoglia38
Romania
2
0
0
Decaro and Buonavoglia38
Hungary
27
0
0
Demeter et al.67; Decaro and Buonavoglia38
Bulgaria
1
0
0
Decaro and Buonavoglia38
Slovenia
1
0
0
Decaro and Buonavoglia38
AFRICA
Tunisia
15
21
14
Touihri et al.269
NORTH AMERICA
United States
1
36
30
Hong et al.106; Kapil et al.129
SOUTH AMERICA
Uruguay
1
0
24
Pérez et al.207
Argentina
9
4
14
Calderon et al.18
ASIA
India
23
4
0
Chinchkar et al.25
India
a
a
3
Nandi et al.177
Taiwan
2
34
0
Wang et al.292
Korea
119
7
0
Kang et al.128; Jeoung et al.122; Yoon et al.300
Japan
4
21
0
Ohshima et al.190
China
27
5
0
Yi et al.298; Zhang et al.303
Thailand
19
7
0
Phromnoi et al.210
OCEANIA
Australia
41
1
0
Meers et al.164
Epidemiology
Pathogenesis
Clinical Findings
Parvovirus Enteritis
Neurologic Disease
Cutaneous Disease
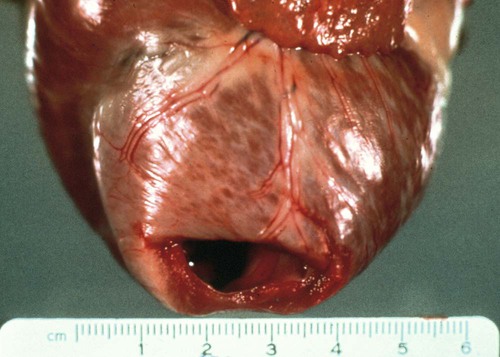
Canine Parvovirus-2 Myocarditis
Bacteriuria
Intravenous Catheter Infection
Diagnosis
Organism Detection
Antibody Detection
Pathologic Findings
Therapy
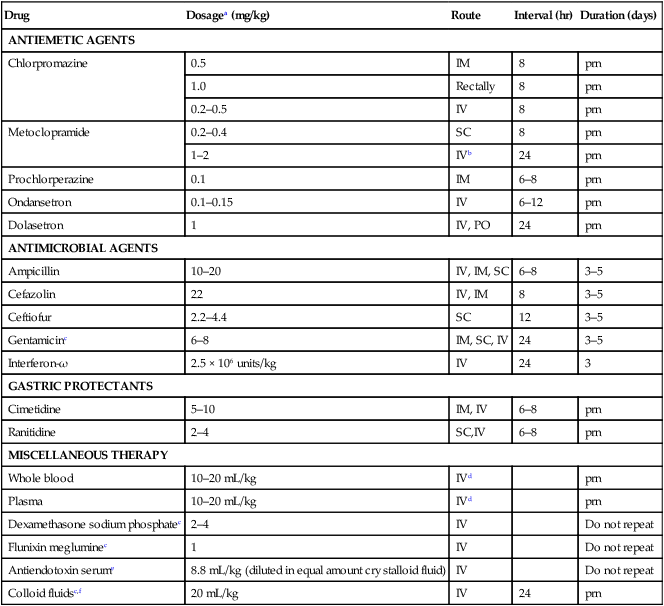
Drug
Dosagea (mg/kg)
Route
Interval (hr)
Duration (days)
ANTIEMETIC AGENTS
Chlorpromazine
0.5
IM
8
prn
1.0
Rectally
8
prn
0.2–0.5
IV
8
prn
Metoclopramide
0.2–0.4
SC
8
prn
1–2
IVb
24
prn
Prochlorperazine
0.1
IM
6–8
prn
Ondansetron
0.1–0.15
IV
6–12
prn
Dolasetron
1
IV, PO
24
prn
ANTIMICROBIAL AGENTS
Ampicillin
10–20
IV, IM, SC
6–8
3–5
Cefazolin
22
IV, IM
8
3–5
Ceftiofur
2.2–4.4
SC
12
3–5
Gentamicinc
6–8
IM, SC, IV
24
3–5
Interferon-ω
2.5 × 106 units/kg
IV
24
3
GASTRIC PROTECTANTS
Cimetidine
5–10
IM, IV
6–8
prn
Ranitidine
2–4
SC,IV
6–8
prn
MISCELLANEOUS THERAPY
Whole blood
10–20 mL/kg
IVd
prn
Plasma
10–20 mL/kg
IVd
prn
Dexamethasone sodium phosphatec
2–4
IV
Do not repeat
Flunixin megluminec
1
IV
Do not repeat
Antiendotoxin serume
8.8 mL/kg (diluted in equal amount crystalloid fluid)
IV
Do not repeat
Colloid fluidsc,f
20 mL/kg
IV
24
prn
Prevention
Immunity After Infection
Immunization and Duration of Immunity
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Canine Viral Enteritis