Chapter 5 CANINE THYROID TUMORS AND HYPERTHYROIDISM
Thyroid nodules in humans are commonly encountered, being particularly frequent among women. The prevalence of people with thyroid nodules in the United States has been estimated to be about 4% of the adult population, with a female:male ratio of 4:1. In young children, the incidence is less than 1%; in persons ages 11 to 18 years, about 1.5%; and in persons over age 60, about 5%. In contrast to the common incidence of thyroid “nodules” in people, thyroid “cancer” is considered rare. According to the Third National Cancer Survey, the incidence of thyroid cancer affecting people within the United States is 0.004% of the populace per year. Thus most thyroid nodules are benign in people (Greenspan, 2001). In contrast to these statistics regarding humans, the statistics for dogs are quite different.
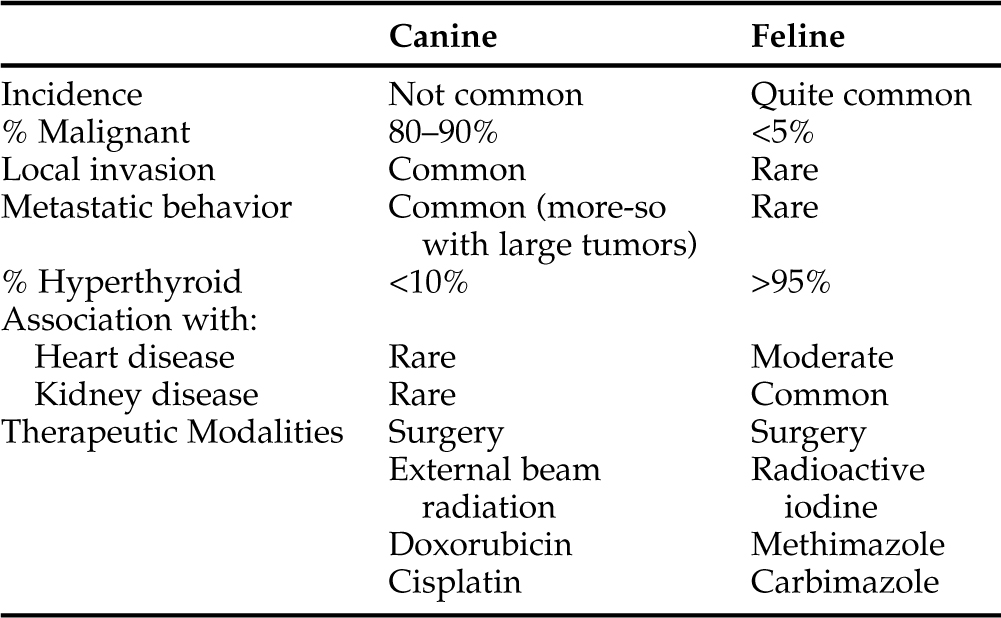
The thyroid gland, made up of two lobes, is not normally palpable in either dogs or cats. However, thyroid tumors are usually easy to feel in both species. Although benign thyroid masses are commonly detected in cats, especially those older than 8 years of age, thyroid masses are only occasionally encountered in dogs. Malignant tumors account for a large percentage of these thyroid nodules in dogs. It has been estimated that thyroid tumors account for approximately 1% to 4% of all canine neoplasms (Priester and McKay, 1980; Birchard and Roesel, 1981; Loar, 1986; Wheeler, 1989; Ogilvie, 1996; Waters and Scott-Moncrieff, 1998). Veterinarians have become well educated regarding thyroid disease in cats. Coincidental with the remarkable incidence of thyroid disease in cats and owner interest in their treatment, has been an increasing number of dog owners being interested in aggressive management of their pets with thyroid tumors. This combination of factors creates an interest for comparing the thyroid conditions affecting the two species. However, significant differences exist in their thyroid diseases (Table 5-1). A tremendous percentage of cats with thyroid disease have benign conditions and hyperthyroidism, whereas dogs with thyroid masses commonly have malignant disease and are usually euthyroid.
Virtually every “general pathology study” published on dogs includes a significant number of dogs with thyroid tumors. In contrast to the relatively recent identification of clinically significant thyroid disease in cats (since 1980), thyroid neoplasia has long been a recognized disorder in dogs. In general, the clinical syndrome caused by thyroid tumors is surprisingly different in these two species. Thyroid tumors in the cat tend to be functioning, noninvasive, relatively small adenomatous masses. In the dog, these tumors tend to be nonfunctioning, invasive, large, carcinomatous masses. In both species, therapeutic measures are warranted, but those that are effective in cats are not usually effective in dogs (see Table 5-1). More than 90% of the clinically detectable thyroid tumors in dogs are carcinomas. This remarkable prevalence concerning malignancy is related, in part, to the fact that most thyroid adenomas in dogs are nonfunctional, they cause no clinical signs, and they are too small to be palpable.
TUMOR CLASSIFICATION
Pathology (Necropsy) versus Clinical Experience
Benign thyroid tumors (adenomas) account for 30% to 50% of thyroid masses identified in studies published by veterinary pathologists (Brodey and Kelly, 1968; Leav et al, 1976). These benign tumors tend to be small, noninvasive, and clinically silent. Therefore, although it has been more than 25 years since these publications first appeared in the literature, it is generally assumed that their results remain valid. Benign tumors are usually described as being incidental findings on necropsy. Clinical studies, however, suggest that almost all thyroid tumors in dogs are malignant and that a significant percentage of those tumors are large, invasive, palpable, and the source of worrisome clinical signs (Mooney, 1998; Lurye and Behrend, 2001; Withrow and MacEwen, 2001). Benign tumors, like normal thyroid lobes, are described as not being palpable. They are mobile, ovoid, and typically involve only one lobe. In contrast, thyroid carcinomas are classically described as large and coarsely multinodular, not mobile, and easily palpable. About one-third of these carcinomas involve both thyroid lobes, and two-thirds are located in one lobe. They are poorly encapsulated and commonly extend into or around the trachea, esophagus, and muscles of the neck. They are highly vascular and may invade local blood vessels.
The Incidentally Discovered Thyroid Mass
Approximately 40% of the thyroid masses that were “incidentally discovered” with cervical ultrasonography have been benign adenomas. After such masses have been described by our radiologists, most clinicians remain unable to palpate them. Approximately 60% of the incidentally identified thyroid masses have been diagnosed with histologic evaluation as being thyroid carcinomas. The frequency of incidentally discovered thyroid masses diagnosed with cervical ultrasonography is expected to increase over the next 5 to 10 years. It is also anticipated that responses to surgery, radiation, and medical management of such tumors will improve, because they will be diagnosed earlier than ever before in the history of our profession. Keep these concepts in mind as you review this chapter. Veterinary medicine, like all such professions, is constantly evolving. The examples provided in the balance of this chapter may represent worst-case scenarios. Management practices and prognoses for thyroid tumors will improve if more of these tumors are diagnosed before they become large, easily palpable, and clinically catastrophic.
NONFUNCTIONING THYROID TUMORS (EUTHYROID OR HYPOTHYROID DOGS WITH NONTOXIC THYROID TUMORS)
Pathogenesis
BACKGROUND.
As with most neoplastic conditions, the exact cause of thyroid neoplasia is not known. Studies have suggested an association between thyroid neoplasia and (1) iodine deficiency or excess, (2) chronic excesses in thyroid-stimulating hormone (TSH) secretion, (3) ionizing radiation, and (4) gene abnormalities and oncogene expression. In addition to these potential pathogenic mechanisms, in some older humans, a long-standing, slowly growing papillary carcinoma may begin to grow rapidly and “convert” to an undifferentiated or anaplastic carcinoma. This condition is usually recognized, because small thyroid masses can be easily detected in people and any sudden change in size can be easily monitored. There may be some similarity between this well-defined process in people and the poorly defined process in dogs. Some dogs will appear to have had a small thyroid mass that “suddenly” begins to expand, dramatically increasing in size. This “late anaplastic shift” is a potential cause of death from papillary carcinoma. Many of these papillary carcinomas secrete thyroglobulin, which can be used as a marker for recurrence or metastasis of the cancer (Greenspan, 2001).
IODINE DEFICIENCY OR EXCESS.
Early epidemiologic data suggested that thyroid cancer in people was more frequent in iodine-deficient areas, but others have contested these findings (Konig et al, 1981). In areas where iodine excess prevails, such as Iceland and Hawaii, a high incidence of thyroid cancer, particularly of the papillary type, has been reported in humans (Verschueren and Goslings, 1992). Experimental data and clinical experience in dogs have neither confirmed nor denied these trends.
THYROTROPIN, THYROID-STIMULATING HORMONE (TSH), AND THYROID GROWTH FACTORS.
The primary factor responsible for maintaining thyroid follicular cell function is TSH (Roger and Dumont, 1982). The role of TSH in thyroid growth is not as obvious, however. There are conflicting opinions regarding the importance of TSH in thyroid neoplasia in humans. In dogs, receptor affinity and concentration, as well as a functional response to TSH, are similar in both normal thyroids and thyroid carcinomas (Verschueren et al, 1992a). Thus some canine thyroid carcinomas appear to retain sensitivity to the growth-promoting effect of TSH, but poor correlation has been demonstrated between TSH binding and TSH dependence in humans (Darbre and King, 1987). Many tumors with TSH binding and adenylate cyclase response did not regress following suppression of serum TSH concentrations with L-thyroxine treatment (Saltiel et al, 1981).
IONIZING RADIATION.
The relationship between ionizing radiation and thyroid cancer has been recognized in humans since the late 1940s and early 1950s. The causative effect of radioiodine on thyroid cancer development was further supported by its high incidence in areas of radioactive fallout, such as the Marshall Islands (Conard, 1984). The short-lived isotopes (132I and 135I) are of greater concern than the more commonly used isotope, 131I (Verschueren, 1992a). Thyroid cancers resulting from radiation exposure are usually well-differentiated thyroid papillary or papillary-follicular carcinomas (Schneider, 1986). Irradiation has been demonstrated to be a cause of thyroid neoplasia in dogs (Verschueren, 1992a).
ONCOGENES.
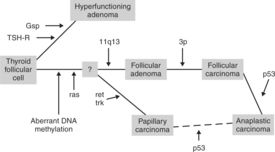
Extensive studies have been conducted on the expression of oncogenes in human thyroid cancers. This research has revealed evidence of gene mutations in both benign and malignant thyroid neoplasms (Fig. 5-1). Activating mutations in the gsp oncogene or TSH-R in the thyroid follicular cell have been associated with increased growth and function. Aberrant DNA methylation, activation of the ras oncogene, and mutation of the MEN1 gene located at 11q13 are associated with benign follicular adenomas. Loss of the 3P suppressor gene may then result in the development of follicular carcinoma, and further loss of suppressor gene P53 may allow progression to an anaplastic carcinoma. Mutations in the ret and trk oncogenes are associated with the development of papillary carcinomas. Again, loss of the P53 suppressor gene may allow progression of this tumor to an anaplastic carcinoma. These hypotheses suggest progressions from benign to malignant tumors or from a “differentiated” carcinoma to an “undifferentiated” (and more lethal) carcinoma. Indeed, pathology specimens from older affected people that had long-standing goiters and recent rapid growth of the masses may demonstrate transition from papillary or follicular carcinoma to anaplastic carcinoma (Suarez et al, 1988; Bos, 1989; Lemoine et al, 1990; Wynford-Thomas, 1997; Komminoth, 1997; Schlumberger, 1998; Hanna et al, 1999; Greenspan, 2001). This phenomenon is referred to as “late anaplastic shift” and is mentioned in the introduction to this section. Similar studies have not yet been applied to canine thyroid neoplasia.
Pathology
RECOGNITION OF MALIGNANT VERSUS BENIGN THYROID.
As is typical of most endocrine tumors, pathologists sometimes have difficulty distinguishing benign from malignant thyroid tumors. Criteria such as cellular atypia and mitotic activity are not consistently reliable markers in discriminating between these two forms of neoplasia. Well-differentiated follicular carcinoma, in some cases, may be histologically distinguished from the benign condition only by demonstration of vascular or capsular invasion (Hedinger et al, 1988; Verschueren and Goslings, 1992). Owing to the recognized prevalence of malignant tumors seen by veterinary practitioners, most pathologists tend to identify thyroid masses that have been biopsied or surgically removed as “malignant until proven otherwise.”
Benign Thyroid Tumors
PHYSICAL APPEARANCE AND CLINICAL FEATURES.
The majority of benign canine thyroid tumors (adenomas) are small, solid, focal lesions that are not usually detected during life. The frequency with which these tumors are identified will be greatly enhanced by the increased use of cervical ultrasonography as a diagnostic tool. It is not understood why small, benign thyroid tumors in the cat tend to be functioning tumors that result in clinical hyperthyroidism whereas similar tumors in the dog are more commonly nonfunctioning or normally functioning. If a dog is diagnosed with a benign, nonpalpable thyroid tumor in our hospital, the most common reason is simple identification of an incidentally discovered mass with cervical ultrasonography. Much less commonly, a dog with a benign tumor may have the same clinical signs associated with any cervical mass: owner observation of the mass, respiratory signs, or difficulty swallowing (Loar, 1986; Lawrence et al, 1991).
Canine thyroid adenomas are often solitary, distinct, slightly soft masses. A well-delineated capsule is seldom noted. Solid adenomas are round or ovoid and measure a few millimeters to several centimeters in diameter. They are usually cream to reddish brown in color and may compress adjacent normal thyroid tissue. Large cystic adenomas can be turgid and filled with an amber or blood-tinged fluid (Belshaw, 1983; Capen, 1985). The central cavities of cystic adenomas have either a smooth or an irregular lining. The physical appearance of canine thyroid adenomas is distinct from the “adenomatous hyperplasia” typically identified in hyperthyroid cats. Although small focal masses similar to those seen in dogs are recognized in cats, the most frequently recognized feline hyperfunctioning thyroid resembles a compressed cluster of grapes involving the entire gland that may be cystic or cavitary upon cutting through the tissue.
HISTOLOGY.
In dogs, most small solid thyroid adenomas are characterized as having follicles that are irregular and either small or large, microscopically. The cystic structures noted in some tumors are lined by dense fibrous tissues from which project fronds of uniform cells arranged in follicular and/or compact cellular patterns. Because cysts are seen in some small adenomas, it is likely that growth is inhibited occasionally by degenerative episodes and these patterns of development are responsible for the final gross and microscopic appearance of these tumors (Belshaw, 1983; Capen, 1985). Although canine thyroid adenomas are well recognized and thoroughly described in the literature, thyroid tumors observed by clinicians should be assumed to be malignant until proven otherwise. Some thyroid tumors may be benign, but the incidence of malignancy and the aggressive nature of these tumors dictate rapid action on the part of the veterinarian. This approach can assure the owners that their pet is being given the best chance for a cure.
Malignant Thyroid Tumors
INTRODUCTION.
Carcinomas of the canine thyroid are usually large solid masses. Their malignant nature is often grossly evident at the time of surgery or necropsy as a result of invasion into adjacent structures. Extension of malignant thyroid tumors into or around the esophagus, trachea, cervical musculature, nerves, and thyroidal vessels is fairly common. However, invasion into the lumen of structures, such as the esophagus or trachea, is extremely unusual. Distant metastasis is quite common. It has been estimated that 40% to 60% of affected dogs have detectable distant metastases at time of clinical admission (Jeglum and Whereat, 1983; Harari et al, 1986; Withrow and MacEwen, 2001). Distant metastases are most likely identified in the pulmonary parenchyma or in regional lymph nodes. By contrast, results of necropsy studies suggest that 60% to 80% of thyroid carcinomas had spread (Capen, 1985; Verschueren et al, 1992b). Of course, the typical necropsy takes place a significant amount of time after the average time of diagnosis. Thus the higher incidence of distant tumor spread is to be expected. In addition, metastases too small to be detected with radiographs, ultrasonography, or other diagnostic aids may be noted histologically.
DEMONSTRATION OF METASTASIS.
Metastasis occurs most commonly to the lungs, retropharyngeal lymph nodes, and liver (Lurye and Behrend, 2001). Lymphatic drainage of the thyroid gland is primarily in the cranial direction, so lymph node enlargement is most likely to be detected cranial and medial to the primary tumor. Occasionally, both ipsilateral and contralateral cervical lymph nodes may be involved. Metastasis to other locations is also possible, including the adrenal gland, kidneys, liver, heart base, spleen, bone and bone marrow, prostate, brain, skeleton, and spinal cord (Harmelin et al, 1993; Verschueren et al, 1992b; Lurye and Behrend, 2001). The differentiation between benign and malignant thyroid neoplasia depends on the answers to two questions: (1) Is there evidence of similar tissue in regional lymph nodes, lungs, or other locations, as well as evidence of local invasion into surrounding structures? (2) What is the microscopic appearance of the tissue? Rarely, small malignant tumors may resemble adenomas. Histologic evaluation is imperative on any thyroid tumor removed by a veterinarian.
HISTOLOGY.
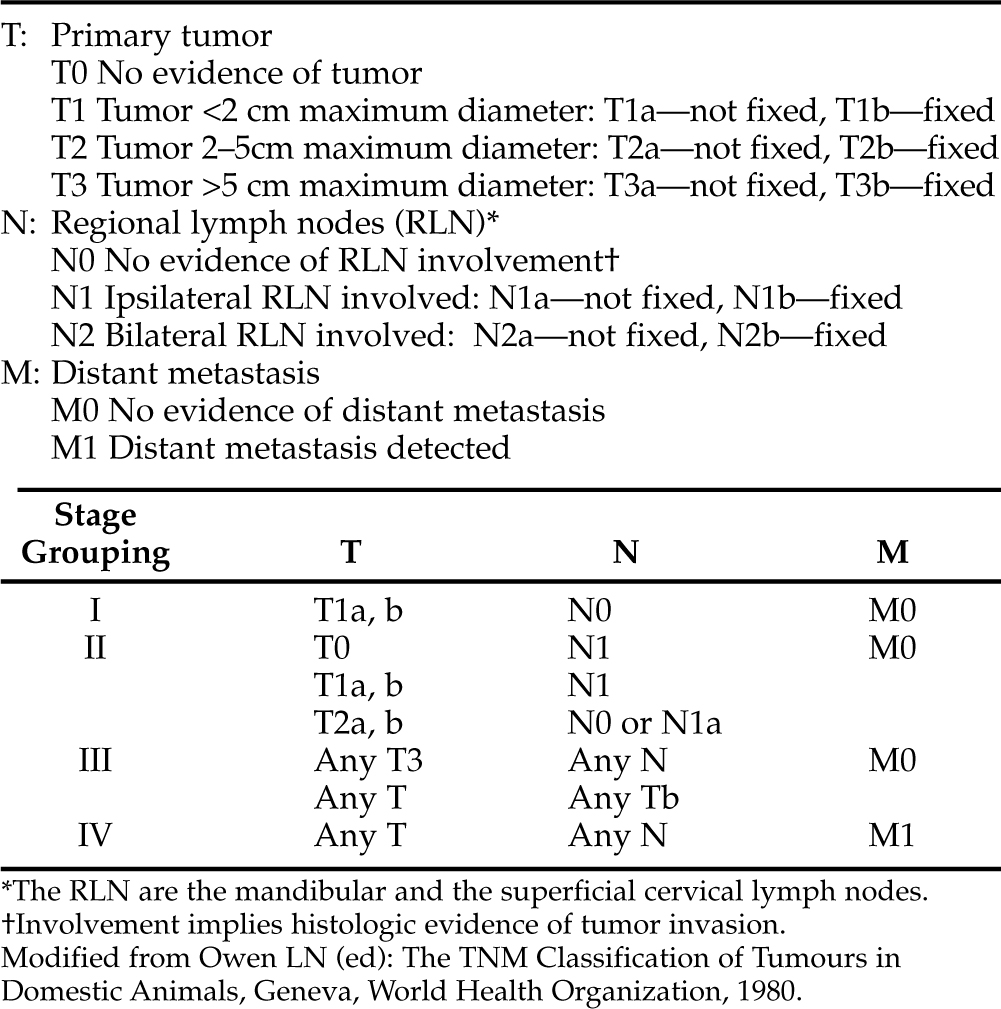
Thyroid tumors of follicular cell origin are usually further subclassified as follicular, compact (solid), papillary, compact-follicular, or undifferentiated (anaplastic), depending on their pattern of growth (Table 5-2). In addition, veterinary oncologists use a clinical staging classification system initially developed by the World Health Organization (Table 5-3). The largest percentage of canine thyroid carcinomas contain both follicular and compact cellular patterns and are classified as “mixed-cellular,” “compact-follicular,” or “solid-follicular” carcinomas. Slightly less common are the pure follicular carcinomas. A smaller percentage of thyroid tumors are pure compact carcinomas. Undifferentiated (anaplastic) tumors are uncommon but recognized in about 10% of dogs with thyroid tumors. Papillary carcinomas are rare in the dog. Thyroid carcinomas may also arise from parafollicular cells (C-cells, medullary carcinomas), but this cell type is uncommon in dogs. As discussed in the next section, medullary thyroid carcinoma has probably been underdiagnosed in the past, and the prevalence of this histologic type of tumor will increase with appropriate use of immunocytochemical stains. The least common thyroid tumors recognized by veterinary clinicians are benign tumors (adenoma). However, as previously discussed, it is anticipated that the prevalence of benign thyroid tumors will rise as cervical ultrasonography provides evidence of incidentally discovered cervical masses. Electron microscopy and immunohistochemistry may be helpful and/or necessary in defining the cell of origin.
TABLE 5-2 HISTOLOGIC DIAGNOSIS OF THYROID TUMORS IN 237 DOGS RECOGNIZED AS HAVING A THYROID MASS ANTEMORTEM
| Tumor Histology | Number | Percent |
|---|---|---|
| Compact follicular carcinomas | 77 | 32 |
| Follicular carcinomas | 56 | 24 |
| Compact (solid) carcinomas | 41 | 17 |
| Undifferentiated (anaplastic) carcinomas | 28 | 12 |
| Parafollicular (C-cell) carcinomas | 20 | 9 |
| Follicular adenoma | 15 | 6 |
| Obvious metastasis or local invasion | 163 | 69 |
IMMUNOHISTOCHEMISTRY.
Immunochemical techniques have refined the pathologic diagnoses of virtually all neoplasms, including those of thyroid origin. Immunohistochemical analysis of thyroid neoplasms for thyroglobulin, calcitonin, calcitonin gene–related peptide (CGRP), and neuron-specific enolase has been studied in dogs and has been demonstrated to contribute to the diagnosis of medullary carcinoma (Moore et al, 1984; Holscher et al, 1986; Leblanc et al, 1991). Studies of these C-cell complexes have demonstrated four different cell types: those that stained for thyroglobulin, calcitonin and CGRP, somatostatin, or no marker. These data suggest the possibility of a common cell lineage for the follicular cell and the parafollicular cell. This finding concurs with the occurrence of mixed follicular and parafollicular tumors in humans, bulls, and horses, but not in dogs (Ljungberg et al, 1983; Ljungberg and Nilsson, 1985; Tateyama et al, 1988; Leblanc et al, 1990; 1991).
It has been accepted that medullary carcinoma may be difficult to distinguish from other thyroid tumors by light microscopy alone. This has probably resulted in a prevalence of underestimation regarding medullary carcinoma among dogs with thyroid malignancy. The incidence was thought to be <5% (Patnaik and Lieberman, 1991; Leblanc et al, 1991); however, when specific immunocytochemical stains were applied to thyroid tumors from dogs, the incidence of medullary thyroid carcinoma was 36%. This is obviously a much higher incidence than previously suggested (Carver et al, 1995). Furthermore, it was thought that histologic classification was not a useful prognostic factor (Brodey and Kelly, 1968; Mitchell et al, 1979; Harari et al, 1986; Klein et al, 1988; Ogilvie, 1996). The long-term prognosis may be better for dogs with medullary carcinoma of the thyroid compared with that in dogs with thyroid carcinomas of follicular origin. Medullary carcinomas have a lower rate of distant metastases, they are well encapsulated versus the incomplete encapsulation typical of follicular carcinomas, and they appear to be easier to surgically remove (83% successful removal of medullary carcinoma versus 52% successful removal of follicular carcinoma; Carver et al, 1995).
MULTIPLE ENDOCRINE NEOPLASIA (MEN) SYNDROMES.
Veterinary medicine has derived benefit, historically, from the experience of physicians in human medicine and from using human beings as our “animal model” of disease. Physicians often have the ability to track family histories relative to disease states and to determine the likelihood that an individual may or may not have certain problems based on “familial” tendencies. This is certainly true of thyroid disease and thyroid neoplasia. Therefore attempts to draw analogies from the human experience and to apply that information to veterinary patients may be helpful. About 80% of people with medullary carcinoma of the thyroid, for example, have “sporadic” disease. By contrast, 20% of those patients have a tumor that can be traced back as a “familial disorder,” that is, one in which their genetic background predisposed them to this condition. Four familial patterns of disease occur with respect to medullary carcinoma of the thyroid. These familial conditions in people should be used to remind veterinary clinicians that a thorough evaluation of any dog is warranted, even after diagnosis of thyroid cancer. This is true because some dogs may have more than one problem. Before investing time, money, and emotion into treating one problem, we should attempt to ensure that a dog does not have other serious disease.
Of the four familial patterns associated with medullary carcinoma of the thyroid in humans, one or more may have analogous conditions in dogs. The four patterns recognized in people are (1) familial medullary thyroid carcinoma without associated endocrine disease (FMTC); (2) multiple endocrine neoplasia syndrome 2A (MEN 2A), consisting of medullary carcinoma, pheochromocytoma, and hyperparathyroidism; (3) multiple endocrine neoplasia syndrome 2B (MEN 2B), consisting of medullary carcinoma, pheochromocytoma, and multiple mucosal neuromas; and (4) MEN 2, consisting of MEN syndrome together with cutaneous lichen amyloidosis, a pruritic skin condition. The genes responsible for these familial syndromes have been mapped to the centromeric region of chromosome 10, which is the location of the ret proto-oncogene (a receptor-like tyrosine kinase gene), and mutations in exon 10, 11, or 16 of this location have been demonstrated in patients with these syndromes (Greenspan, 2001). If medullary carcinoma is diagnosed in a person, it is recommended that family members be screened for that tumor and that the patient and family members be screened for any of the other conditions found in MEN 2. In addition to tests designed to detect abnormalities suggestive of medullary carcinoma, gene mutations can be demonstrated in DNA from peripheral white blood cells by using polymerase chain reaction (PCR), restriction fragment-length polymorphism (RFLP), and family unit linkage analysis to identify gene carriers. In this manner, families can be screened for the carrier state, as well as for early diagnosis and treatment of serious disease states.
Dogs occasionally have tumors in several different endocrine organs (including the thyroid) simultaneously (Table 5-4). The answer as to whether these dogs have familial disorders analogous to the well-defined MEN syndromes in human beings awaits genetic study. It is suspected that many of these dogs simply have coincidental multiple problems. However, it is interesting to note that we have diagnosed seven dogs with concurrent medullary carcinoma of the thyroid, pheochromocytoma, and primary hyperparathyroidism due to a parathyroid adenoma. This does fit the criteria for MEN 2A. Also, a number of other dogs with thyroid tumors in our series have shown evidence of other glandular neoplasias (see Table 5-4). These 63 dogs include 27 different breeds. Multiple endocrine tumors do exist in dogs and cats, emphasizing the value of complete diagnostic evaluations even when a tumor, such as a thyroid mass, is palpable and recognized easily.
TABLE 5-4 HISTOLOGIC EVIDENCE OF MULTIPLE ENDOCRINE NEOPLASIA (MEN) SYNDROME IN 63 DOGS WITH THYROID TUMORS
| Tissue Description | Number of Dogs |
|---|---|
| Thyroid adenoma and chemodectoma | 2 |
| Thyroid follicular carcinoma and adrenocortical adenoma | 4 |
| Thyroid follicular carcinoma and adrenocortical carcinoma | 4 |
| Thyroid follicular carcinoma, pituitary adenoma, and adrenocortical hyperplasia | 12 |
| Thyroid follicular (3) or compact-follicular carcinoma (3) and parathyroid hyperplasia/adenoma | 6 |
| Thyroid compact-follicular carcinoma, pituitary adenoma, and adrenocortical hyperplasia | 11 |
| Thyroid follicular adenoma, pheochromocytoma, pituitary adenoma, and adrenocortical hyperplasia | 3 |
| Thyroid follicular adenoma and adrenocortical adenoma | 4 |
| Thyroid parafollicular (C-cell) carcinoma, pituitary tumor, and pheochromocytoma | 4 |
| Thyroid parafollicular (C-cell) carcinoma, pheochromocytoma, and parathyroid adenoma | 7 |
| Thyroid compact-follicular carcinoma and parathyroid adenoma | 6 |
SIGNALMENT.
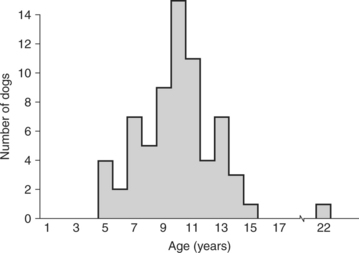
Thyroid tumors in the dog typically develop in middle-aged and older animals. The average age for dogs with thyroid tumors, benign or malignant, is approximately 10 years. The age range is quite wide, but almost all dogs with thyroid tumors are 5 years of age or older (Fig. 5-2). One study conducted within a small colony of Beagles demonstrated an age-specific incidence of thyroid tumors of 1.1% per year in dogs 8 to 12 years of age and 4.0% per year in dogs 12 to 15 years of age (Haley et al, 1989).
There is no obvious gender predilection for thyroid neoplasia in the dog (Harari et al, 1986). By contrast, the incidence of human thyroid cancer is about four times greater in women than in men at most ages (Greenspan, 2001). The breeds thought to be at increased risk appear to include Boxers, Beagles, and Golden Retrievers (Harari et al, 1986; Verschueren, 1992), although our experience is slightly different, with mixed-breed dogs and Labrador Retrievers being most commonly afflicted. As seen in Table 5-5, Beagles, Boxers, and Golden Retrievers were among the 42 breeds represented in our series. Breed incidence in dogs with thyroid tumors, in our experience, tends to correlate with breed popularity.
TABLE 5-5 BREED DISTRIBUTION OF 237 DOGS WITH THYROID TUMORS
| Breed | Number | Percent |
|---|---|---|
| Mixed-Breed Dogs | 32 | 13 |
| Labrador Retriever | 22 | 9 |
| Golden Retriever | 17 | 7 |
| Boxer | 15 | 6 |
| Australian Shepherd | 14 | 6 |
| German Shepherd Dog | 14 | 6 |
| Poodle | 14 | 6 |
| Dachshund | 12 | 5 |
| Rottweiler | 12 | 5 |
| Beagle | 12 | 5 |
| Doberman Pinscher | 10 | 4 |
| 32 other breeds (<10 cases each) | 63 | 27 |
CLINICAL SIGNS.
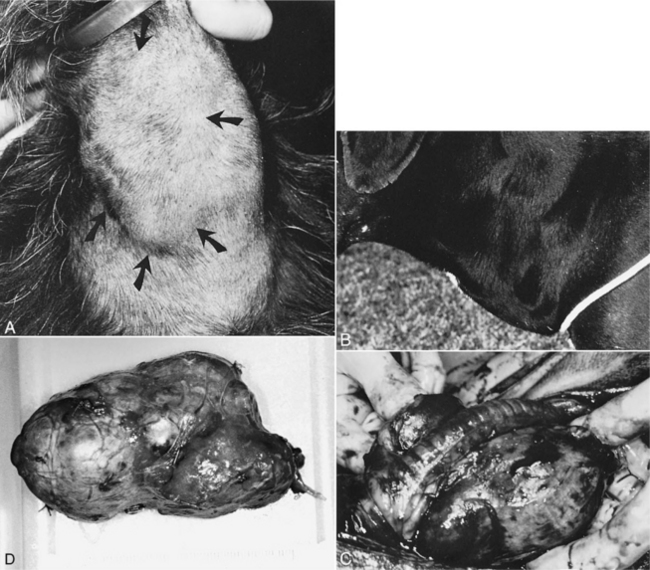
Dogs with nontoxic (not hyperthyroid) thyroid tumors are usually brought to veterinarians because the owners have seen or felt a mass in the neck or because the mass is causing clinical signs. The signs reported by different authors vary slightly. Without doubt the most frequent owner concern is feeling or sometimes seeing a midcervical or ventrocervical mass. Owners, while petting and scratching their dogs, may simply note the presence of a “swelling” in the throat area (Fig. 5-3). The mass may be unilateral or bilateral in location. Most thyroid tumors are felt to be just below the area of the larynx, but larger tumors may extend (common) or descend (uncommon) closer to the thoracic inlet.
Because most affected dogs remain healthy for some time, the swelling in the neck caused by tumor growth is often noted to develop slowly. In more than 75% of our dogs that were diagnosed antemortem as having a thyroid tumor, either the swelling was the only reason for seeking veterinary care or the swelling was pointed out by a veterinarian during an examination for some other problem (Table 5-6). Other clinical signs include coughing, rapid breathing (especially at times of rest), respiratory distress, difficulty in swallowing (dysphagia), alteration in the sound of a normal bark (dysphonia), weight loss, listlessness/depression, vomiting, regurgitation, anorexia or an obvious decrease in appetite, polydipsia, polyuria, hyperactivity, diarrhea, increased appetite, facial edema, and apparent pain or discomfort (see Table 5-6). Not surprisingly, the length of time between owner observation of the mass or clinical sign(s) and presentation of the dog for veterinary care is highly variable. In our series, some dogs were seen as quickly as the same day a mass was discovered, whereas others were not seen until as long as 36 months later. The average length of time was within 2 to 6 weeks.
TABLE 5-6 OWNER-OBSERVED SIGNS IN 237 DOGS WITH THYROID TUMORS
| Sign | Percent of Dogs |
|---|---|
| Visible mass in neck | 78 |
| Coughing | 34 |
| Rapid breathing (even at rest) | 32 |
| Dyspnea (difficulty breathing-distress) | 28 |
| Trouble swallowing (dysphagia) | 23 |
| Change in bark (dysphonia) | 14 |
| Weight loss | 14 |
| Listlessness/Depression | 13 |
| No observed signs | 12 |
| Vomiting/Regurgitation | 11 |
| Anorexia/Decrease in appetite | 11 |
| Polydipsia/Polyuria | 10 |
| Hyperactivity | 9 |
| Diarrhea | 9 |
| Increased appetite | 3 |
| Facial edema | 2 |
| Apparent cervical discomfort | 2 |
PHYSICAL EXAMINATION.
Most thyroid masses are firm, asymmetrical, irregular in shape, and nonpainful. If both thyroid lobes are affected, the masses tend to be irregular and asymmetrical. Most thyroid tumors are located close to the typical normal thyroid region (at and ventral to the laryngeal area) in the neck and are not as ventral in location or as freely movable in the subcutaneous space as those in cats (see Fig. 5-3). Usually, the thyroid mass is nonmovable and obviously well embedded into surrounding tissue. It is usually not possible to palpate the interior or medial surface of the mass because of local invasion. An irregular shape is not always diagnostic of carcinoma, but an immovable mass usually implies local invasion and should raise a strong suspicion for malignancy at the time of examination.
Inspiratory and expiratory dyspnea has been identified in several dogs. Only a few dogs have been obviously cachectic (Fig. 5-4), depressed, or dehydrated. The noncachectic dogs are usually alert and active and have good mucous membrane color, normal capillary refill time, and no fever. A dry, lusterless hair coat is common, but alopecia is rare. Heart sounds are normal, and examination of the mouth, eyes, ears, and abdomen is unremarkable. Submandibular lymph nodes may be enlarged as a result of tumor spread or lymphatic obstruction.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree