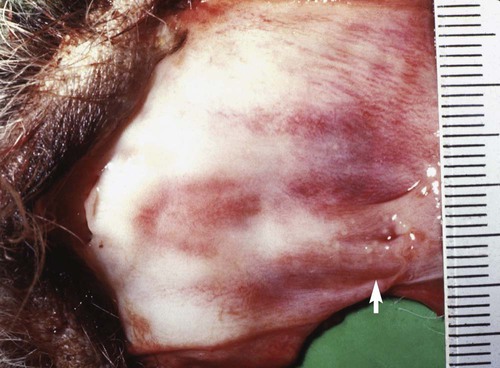
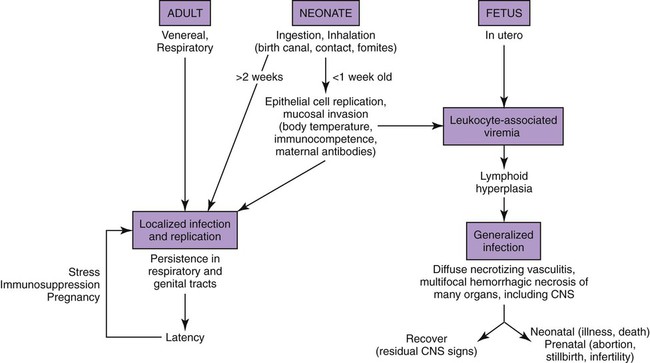
Canine herpesvirus (CHV) has a worldwide distribution, with biologic and pathogenic properties similar to those of α-herpesviruses affecting other species. α-Herpesviruses are cytocidal, causing tissue necrosis and localized mucosal or generalized systemic infection in young or immunosuppressed animals. Recovery is associated with a lifelong latent infection, usually localized to nerve ganglia. CHV has a relatively narrow host specificity compared with other members of the α-herpesviruses. CHV infects only dogs or canine tissue cells. Specific receptors on the cell surface have been identified that contribute to this specificity.3,34 Although an antigenic relationship to human herpes simplex virus has not been confirmed, CHV shares approximately 51% genetic homology with feline herpesvirus type 1 (FHV-1).51 An antigenic relationship between the canine and feline herpesviruses has also been confirmed in immunoblots with polyvalent or monoclonal antibodies.21,60 One differentiating feature between these viruses is their glycoprotein D hemagglutinins, which offer selective adherence to cells from their like species and may partially explain the species specificity of these viruses.30 Less defined immunologic relationships exist between CHV and herpesviruses isolated from harbor seals (Phoca vitulina) and equine herpesviruses 1 and 4.12,19 Analysis of CHV isolates by restriction endonuclease cleavage of viral DNA revealed differences in the viruses isolated from unrelated individuals, but cleavage patterns of isolates derived from members of the same litter were indistinguishable.61 CHV has a restricted host range and appears to infect only domestic and wild Canidae or canine cell cultures, especially primary or secondary kidney or testicular cells. The virus causes rapidly spreading, highly destructive cytopathic clear plaque effects in cell cultures with formation of type A intranuclear inclusions; some isolates induce syncytial cell formation (see Viral Isolation in this chapter). Although CHV has not been reported in cats, it is unclear whether an FHV-1 isolate from a pup with a distemper-like syndrome and pancreatic atrophy causes canine infections. Young pups given large (more than 106) doses of this FHV-1 virus by multiple routes failed to develop clinical illness or histopathologic changes.21 Cross-species infections with herpesviruses may be established in unnatural hosts through artificial means. A nonpathogenic strain of human herpes simplex virus 1 was injected into the brains of normal dogs, establishing a latent infection with no pathologic changes.54 Equine herpesvirus 9 is a neurotropic virus that infects horses. Dogs challenged intranasally with equine herpesvirus 9 developed a fulminant nonsuppurative encephalitis.64 The forebrain was predominantly affected, and virus was detected in neurons. Dogs also had bronchopneumonia and clinical signs of weight loss, fever, anorexia, and neurologic symptoms. The clinical significance of natural infections is unknown. CHV is not stable in the environment, but it is maintained in nature by persistence in its canine host and direct spread from infected animals. CHV is a temperature-sensitive virus, with optimal replication at temperatures less than 37° C. It persists in the ganglionic and lymphoid tissues of the oronasal and genital mucosae. As with other herpesviruses, lifelong latent infections are typical. When infections reactivate, CHV replicates in the cooler temperatures of the mucous membranes, and shedding occurs. Virus reshedding occurs sporadically, usually when animals are stressed, such as those in high population densities, those being transported, those that are pregnant, or those that are receiving immunosuppressive therapy. Transmission occurs through direct contact with mucosal secretions from the respiratory or genital tracts of infected animals. Data from serosurveys in wild canid populations indicate a ubiquitous natural exposure.1,47 Serologic surveys in domestic dogs have ranged from 30% to as high as 100% in some kennels.10,14,38,42 A higher prevalence of seroreactivity exists in kenneled dogs than in household pets. Despite these high rates of exposure, clinical disease may not be evident. However, significantly higher CHV-1 serum antibody titers and prevalence of positive results have been found in dogs in breeding kennels with reproductive problems and infectious respiratory disease, or when poor hygiene was practiced, as compared to those without.11,48,48 In a temporal study of serologic reactivity among dogs in breeding kennels, entering bitches that originally had seronegative results seroconverted after entry, whereas some of those within the kennel had seropositive results become negative during the monitoring period.48 Reproductive disorders that developed could not be correlated with changes in the serologic status of the bitches. Furthermore, results of polymerase chain reaction (PCR) were negative on vaginal and nasal swabs and buffy coat specimens collected from actively infected dogs. Postnatally infected pups that are born in these endemic environments are exposed at birth but may have no symptoms. Factors that predispose neonatal pups to generalized infection are hypothermia and poorly developed immune systems. Newborn puppies can acquire CHV infection in utero, from passage through the birth canal, from contact with infected littermates, from oronasal secretions of the dam, or from fomites (although this is rare). A systemic cell-associated viremia is possible in immunodeficient or immunosuppressed hosts. Neonatal puppies experimentally infected when they are younger than 1 week are particularly susceptible to fatal generalized infections; dogs older than 2 weeks at the time of infection are relatively resistant and generally develop mild or inapparent clinical illness. Virus replication in older dogs is restricted to the nasopharynx, genital tract, tonsils, retropharyngeal lymph nodes, bronchial lymph nodes, conjunctival tissues, and occasionally lungs. Virus can be harbored in the lymphoid tissues such as the tonsils, parotid salivary gland, and sensory ganglia.7,31 The inherent immunity in a given breed of dog, or canid species, may play an important role in protection against herpesvirus infections. Adult European red foxes (Vulpes vulpes) developed systemic and respiratory illness after experimental intravenous challenge of a viral strain that produced only mild signs in adult domestic dogs.43 Mucosal immune mechanisms are likely important in natural infection because adult foxes given the same challenge by mouth (per os) had no clinical disease but seroconverted.43 When experimentally infected foxes were given repositol glucocorticoids as immunosuppression, 4 months or 11 months postinoculation (PI), CHV was not detectable in blood leukocytes or mucosal secretions or spread to in-contact control animals.44 Therefore, reactivation of infection could not be demonstrated; however, CHV DNA was detected in the trigeminal ganglia at necropsy, 18 months PI, in all of the experimentally infected foxes. In experimental studies, route of inoculation has also been shown to be important in determining the spread and tissue localization of virus within the body. Dogs given CHV intranasally or both intranasally and intravenously had virus isolated from nasal secretions.31 Dogs given an intravaginal inoculation had virus isolated in both nasal and vaginal secretions. Two to 4 months after inoculation, necropsies were performed on the animals, and CHV could not be cultured from any tissues. However, using PCR, the CHV genome was found in trigeminal ganglia and retropharyngeal lymph nodes, regardless of the inoculation route. Convalescent dogs also had the viral genome in the lumbosacral ganglia, tonsils, and mediastinal and hypogastric lymph nodes.31 However, the CHV genome could not be detected in peripheral blood mononuclear cells. Within the trigeminal and lumbosacral ganglia and associated lymph nodes, virus is localized in the neurons or intranuclearly in lymphocytes during this quiescent period. These are likely sites for latency, and recrudescence results in virus replicating and shedding from the respiratory and genital mucosae. Although neonatal infection usually is acquired at or soon after birth, transplacental transmission can also occur. The effects of transplacental infection with CHV depend on the stage of gestation at which infection occurs (Fig. 5-1). Infertility and abortion of stillborn or weak pups with no clinical signs in the dam have been reported. Although some puppies can survive such in utero infections and appear normal after delivery by cesarean section, others harbor the virus inapparently in their tissues. However, most pups develop systemic herpesvirus infection within 9 days of birth. After oronasal exposure, CHV is first detected in the nasal epithelium and pharyngeal tonsils (Fig. 5-2). Primary replication occurs in epithelial cells and mucosa within 24 hours PI. The virus then enters the bloodstream by way of macrophages. Intracellular viremia results in viral spread throughout the body within 3 to 4 days after inoculation. Virus localization in the mononuclear phagocytic cells of the lymph nodes and spleen results in cell-to-cell spread and lymphoid hyperplasia and necrosis. Progressive multifocal hemorrhagic necrosis occurs in several organs; the highest concentrations of virus are found in the adrenal glands, kidneys, lungs, spleen, and liver. Multifocal hemorrhage associated with necrotic lesions may be related to the vasculitis and marked thrombocytopenia that occurs during infection. Thrombocytopenia can result from disseminated intravascular coagulation associated with widespread vascular endothelial damage and tissue necrosis. Occasionally herpesviruses have been isolated from papulovesicular lesions of the canine genital tract; such lesions may be recurrent episodes in previously infected bitches. With CHV, localized genital or respiratory infections and viral shedding can occur in the presence of circulating antibodies. Infection of the genital tract generally appears to be asymptomatic or limited to vaginal hyperemia with hyperplastic lymphoid follicles. Genital localization of the virus in bitches may be a means of venereal transmission of the virus, but it is most important as a source of infection for pups at birth. Spread of CHV from seropositive males to susceptible females at the time of breeding does not appear to be a significant mode of transmission, although such a mechanism is believed to occur. PCR has shown persistence of the virus in the lumbosacral ganglion, with presumed recrudescence and mucosal replication with subsequent shedding by the genital mucosae.7,31 Experimentally, tracheobronchitis has been produced in dogs infected with an isolate from naturally infected dogs.20 Field evidence suggests that CHV is one viral cause of canine infectious respiratory disease complex (see Chapter 6). In a temporal study in shelter dogs, CHV was found in 9.6% of bronchoalveolar lavage and 12.8% of tracheal samples of necropsied dogs suffering from endemic respiratory illness.15 Compared to other incriminated pathogens for which the dogs had received vaccination, CHV-associated illness was usually more severe, and its isolation rate was usually highest 3 to 4 weeks after the dog’s introduction into the kennel. This later onset of CHV-associated illness may have been related to delayed exposure after entry, prolonged incubation, or reactivation of latent infections in dogs suffering from co-infecting pathogens. Although of uncertain significance, CHV has been recovered from the lungs of dogs with distemper and from dogs with acute conjunctivitis. Neonates that recover from CHV infections or older dogs that have subclinical infections have periodic episodes of viral recrudescence in their oronasal secretions. Viral latency has been demonstrated in intranasally-infected dogs for as long as 6 months after infection, with recrudescence occurring within 1 week after treatment with glucocorticoids or anti-lymphocyte serum.8 Recrudescence of latent virus also has been demonstrated in seropositive adults after being exposed to seronegative juveniles, suggesting a stress mechanism for CHV transmission that is similar to that of FHV infection. Reactivation of latent infections with asymptomatic shedding of virus from nasal, oral, ocular, and vaginal secretions occurred in most bitches given repeated high immunosuppressive doses of glucocorticoids.39 Recrudescence is a plausible explanation for subclinical persistence and the rare recurrences of abortions, fetal infections, or neonatal illnesses. It also serves as a mode of viral transmission to susceptible dams, especially when they are in a kennel for breeding. Dogs older than 3 to 4 weeks develop a mild or inapparent upper respiratory infection as a result of CHV. Severe pneumonic manifestations with systemic infection have been identified in pups as old as 8 weeks5 and older.18 CHV has been recognized as causing endemic canine infectious respiratory disease in kennels (see earlier discussion, Adult Respiratory Infection under Pathogenesis, and Chapter 6). Signs of systemic infection are rare in older pups; however, vomiting, anorexia, depression, serous ocular discharge, hepatomegaly, and sudden death have been reported in naturally infected 8-to 10-week-old coyote pups (see Chapter 92, and later in this chapter, for a discussion of ocular lesions). Infections with CHV can cause genital infections and visible lesions in adult dogs. Primary genital infections in older bitches are characterized by lymphofollicular lesions, variable degrees of vaginal hyperemia, and occasionally petechial or ecchymotic submucosal hemorrhage (Fig. 5-3). No discomfort or vaginal discharge is noted in affected pregnant bitches, even in those who had abortions or stillbirths. Vesicular lesions have been noted during the onset of proestrus and regress during anestrus. Male dogs, with similar lesions over the base of the penis and preputial reflection, may have a preputial discharge.
Canine Herpesvirus Infection
Etiology
Epidemiology
Pathogenesis
In Utero Infection
Systemic Neonatal Infection
Adult Genital Infection
Adult Respiratory Infection
Clinical Findings
Neonatal Puppies
Older Puppies and Adult Dogs
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Canine Herpesvirus Infection