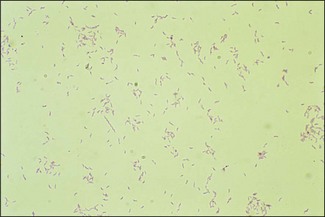
Chapter 24 The Campylobacter species are members of the family Campylobacteraceae, together with Arcobacter species and Bacteroides ureolyticus. They are thin, curved, Gram-negative motile rods. The cells are 0.2–0.5 µm in width and when daughter cells remain joined, S-shaped, seagull-shaped and sometimes long spiral forms may be seen (Fig. 24.1).They are motile by a single polar flagellum, oxidase-positive, catalase-variable and most are microaerophilic. They grow best on nutritious basal media supplemented with 5–10% blood under reduced oxygen tension. Members of the Campylobacteraceae do not utilize carbohydrates but obtain energy from amino acids or intermediates of the tricarboxylic acid cycle. Some species of Campylobacter are thermophilic, growing best at 42°C, the most important pathogen in this category being C. jejuni. The most significant animal pathogens are C. fetus subsp. fetus, C. fetus subsp. venerealis and C. jejuni. Figure 24.1 Campylobacter fetus subsp. fetus in a DCF-stained smear from culture showing the curved rods and ‘seagull’ forms characteristic of the genus (× 1000). Members of the genus Arcobacter species are emerging animal and human pathogens (Snelling et al. 2006). They are described as aerotolerant Campylobacter-like organisms although recent phylogenetic analysis has cast doubt on whether the genus should be included in the Campylobacteraceae (Miller et al. 2007). Arcobacter species have been isolated from cases of abortion in animals and diarrhoea in humans. The Campylobacter species are worldwide in distribution. Many species are commensals on the mucosa of the oral cavity and intestinal tract of animals and birds. Campylobacter fetus subsp. venerealis occurs in the prepuce of bulls and in the genital tract of cows in herds where bovine genital campylobacteriosis is, or has been, present. There are also some non-pathogenic species that are saprophytes in the environment. Arcobacters may be found in the faeces of many animal species and it has been suggested that poultry may act as reservoirs of this organism. However, genomic studies suggest that A. butzleri may be a free-living, water-borne organism (Miller et al. 2007). Helicobacters are found in the gastrointestinal tracts of many animals and birds. Transmission of many of the Campylobacter species including C. fetus subsp. fetus, is by the faecal–oral route. Campylobacter fetus subsp. venerealis is transmitted by coitus and infection of the female genital tract may lead to metritis with resulting death and resorption of the embryo (infertility), or occasionally to abortion. The diseases associated with the Campylobacter and related species and/or their commensal status, are given in Table 24.1. There are two subspecies of C. jejuni, subspecies jejuni and subspecies doylei. Subspecies jejuni is pathogenic whereas the role of subspecies doylei in animals and humans is unclear. Pathogenic isolates of C. jejuni show several virulence attributes. Motility, chemotaxis and temperature stress responses are important pathogenic factors as mutants lacking these characteristics are unable to colonize the intestines of chickens (Konkel et al. 1998, Fields & Swerdlow 1999). Flagella play a role in adhesion in addition to motility. Other adhesins involved in both adherence and invasion have been identified (Konkel et al. 1997, Pei et al. 1998). Following adhesion, C. jejuni is internalized into the epithelial cells lining the intestine and may replicate once inside its membrane-bound compartment within the cell. Exocytosis can occur at the basolateral surface of the cell. Mechanisms of disease production are unclear although the organism is known to produce a cytolethal-distending toxin. Outer membrane constituents lipo-oligosaccharides and polysaccharides are involved in adhesion, endotoxicity and serum resistance. In addition, phase variation may be important in immune evasion. The pathogenic mechanisms of C. fetus in the production of reproductive loss remain largely unknown. Genomic studies suggest that this species possesses many of the same genes encoding virulence attributes as C. jejuni, including genes encoding outer membrane proteins, flagella, regulatory and secretion systems (Moolhuijzen et al. 2009). In addition, a genomic island encoding the components of a type IV secretion system has been identified in C. fetus subspecies venerealis only (Gorkiewicz et al. 2010). Extensive studies have been conducted on the surface layer (S-layer) proteins which this species produces. The S-layer, which is a paracrystalline protein structure external to the bacterial outer membrane, confers resistance to complement-mediated killing in nonimmune serum. In addition, eight antigenic variants of the protein can be expressed by the bacterium and this may delay the host immune response (Grogono-Thomas et al. 2003). Some of the virulence attributes of C. jejuni and C. fetus are given in Table 24.2. Virulence mechanisms of Arcobacter and Helicobacter species in animals are currently unclear. Table 24.3 summarizes the specimens required from the various clinical conditions for the diagnosis of Campylobacter species. Transport medium should be used for the collection of specimens for the isolation of C. fetus. A study by Monke et al. (2002) suggests that Weybridge transport enrichment medium in combination with Skirrow agar and culture within four hours of sampling gives enhanced sensitivity of isolation. See Appendix 2 for composition of transport and culture media. Table 24.3 Suitable specimens for detection of Campylobacter species Other Campylobacter species can be demonstrated in ways similar to those described above and detailed descriptions of these techniques can be found in the OIE Manual (Anon. 2008). Culture media and isolation procedures for C. fetus, C. jejuni and other Campylobacter species are presented in detail in the OIE Manual (Anon. 2008). 1. Campylobacter fetus (both subspecies): cervical mucus and preputial washings can be passed through a 0.65 µm membrane filter to reduce contamination. Foetal abomasal contents and filtrates are inoculated onto a nutritious base (Brucella, Columbia or brain heart infusion agars) supplemented with 5–10% blood. The medium can be made selective by the addition of polymyxin B sulphate (2 units/mL), novobiocin (2 µg/mL) and cycloheximide (20 µg/mL). As cycloheximide has toxic effects and is likely to become unavailable in future years, it can be replaced by 10 µg/mL of amphotericin B (Aspinall et al. 1993). A number of selective media for the isolation of C. fetus have been described including Skirrow agar and Clark’s selective medium (Anon. 2008). Non-selective blood agar should be inoculated simultaneously for the possible isolation of other pathogens. The plates are incubated at 37°C for four to six days in a microaerophilic atmosphere containing 6% O2, 10% CO2, and 85% N2. These atmospheric conditions must be adhered to strictly and can be attained by one of the following methods (the first two methods are preferable): • Filling an anaerobic/CO2 jar with the specified ready-mixed gas in a cylinder obtained from a commercial gas supplier. • Using special gas-generating envelopes such as CampyPak™ II (BBL) or BR56/BR60 (Oxoid) with a palladium catalyst in an anaerobic/CO2 jar. • An anaerobic gas-generating envelope in the jar without a catalyst. • Incubating a plate inoculated with a facultative anaerobe together with the plate for isolation of Campylobacter species. Both plates are placed in an airtight plastic bag.
Campylobacter, Arcobacter and Helicobacter species
Genus Characteristics
Natural Habitat
Pathogenesis and Pathogenicity
Laboratory Diagnosis
Specimens
Clinical entity
Specimens
Bovine infertility
(C. fetus subsp. venerealis)
Vaginal or cervicovaginal mucus from females collected on a herd basis (10–20 animals). Specimens can be collected by swabbing, suction or by washing the vaginal cavity; a sterile speculum should be used to ensure collection of good-quality samples. Samples should always be placed in transport medium
Preputial mucus or smegma can be obtained by scraping, suction or preputial washing. Semen samples are suitable also. Transport medium is recommended
Bovine and ovine abortion
(C. fetus subsp. venerealis, C. fetus subsp. fetus and C. jejuni)
Foetal abomasal contents preferred although the placenta, liver and lungs of the foetus may be submitted. Further samples from dam and foetus to exclude other infectious agents
Diarrhoea
(C. jejuni and others)
Rectal faeces. If swabs are used or if culture of faecal samples will be delayed, samples should be stored in transport media such as Amies, Cary–Blair or Stuart media
Direct microscopy
Isolation procedures
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Campylobacter, Arcobacter and Helicobacter species
Only gold members can continue reading. Log In or Register to continue