Chapter 26 BRUCELLOSIS AND TRANSMISSIBLE VENEREAL TUMOR
BRUCELLOSIS
Infectious Agents
INFECTION WITH B. MELITENSIS, B. ABORTUS, AND B. SUIS.
These three species of Brucella have been reported to cause infections in the dog through either natural or experimental exposure. Natural infection occurs with ingestion of contaminated milk, meat, or aborted fetuses or the fetal membranes of infected livestock. Dogs appear to be relatively resistant to brucellosis because infection may result in no, few, or mild clinical signs. These infections may persist without clinical signs for varying periods after confirmation of the diagnosis (Pidgeon et al, 1987). Infected dogs usually show positive results on the conventional serologic tests used to diagnose brucellosis in livestock, such as the card or tube agglutination test (Barr et al, 1986; Nicoletti, 1989). Natural and experimentally induced infections have been transmitted to livestock (Kormendy and Nagy, 1982). Infected dogs therefore have the potential to infect not only cattle but also humans, and they pose a threat of longer duration of disease transmission in cattle than was once assumed (Johnston et al, 2001).
INFECTION WITH B. CANIS.
Natural infection with B. canis appears to be limited to the Canidae family. Humans are accidental hosts, and infections are usually relatively mild compared with the clinical signs in humans infected with other Brucella species. Livestock, primates, cats, and rabbits are quite resistant to experimental infection (Greene and George, 1984; Nicoletti, 1989).
Transmission of Brucella canis
TRANSMISSION BETWEEN DOGS.
Infection can occur readily across mucous membranes, causing dogs to be infected by oronasal, conjunctival, or vaginal exposure. Transmission occurs readily when an uninfected bitch is mated with an infected male. Males can also acquire the disease from the female (Greene and George, 1984). Interestingly, in one study, when infected and uninfected dogs of the same gender were housed together for as long as 10 months, transmission did not occur (Hubbert et al, 1980). This suggests that urine and mucous membrane secretions are less important factors in the natural transmission of the bacteria. However, in a subsequent study, transmission between sexually mature male dogs was reported to occur after 4 to 6 months of cohabitation (Carmichael and Joubert, 1988).
Dogs without clinical signs can harbor Brucella organisms for prolonged periods. The time from exposure to bacteremia is usually 21 days (Meyer, 1983). After initiation of bacteremia, the organisms can become localized, causing continuous or recurrent bacteremia that lasts a few months to 3 to 4 years. Bacteria can be localized in the prostate or epididymis (or both) of an asymptomatic male. These focal infection sites can serve as a source of widespread dissemination if such a male is actively used in breeding. Venereal transmission probably occurs readily, even when low numbers of organisms are shed. Venereal transmission appears to occur most frequently when infected males are bred to susceptible females and somewhat less often when susceptible males are bred to infected females (Carmichael and Joubert, 1988).
In a kennel situation, a Brucella-infected aborting bitch is highly dangerous to Brucella-free dogs. Aborted placental tissues and fluids may contain huge numbers of organisms. Spread throughout a kennel is rapid, and persistent discharge of infected uterine secretions may continue for 4 to 6 weeks after a single abortion. Milk from infected bitches, which has been shown to contain abundant numbers of organisms, serves as an additional environmental contaminant. The effect on a breeding kennel can be devastating. With the potential for asymptomatic and prolonged bacteremia, blood transfusions also function as vectors for dissemination of brucellosis (Zoha and Walsh, 1982).
TRANSMISSION TO HUMANS.
During the years since the initial isolation of B. canis, an extremely small number of cases have been reported in humans. Most of these individuals were laboratory personnel or kennel attendants who had repeated and/or massive exposure to the organism. However, a few people who had contact only with infected pets have contracted the organism. Therefore it is wise to advise but not frighten owners regarding the zoonotic potential of the disease (Johnston et al, 2001).
Clinical Signs of Brucella canis
NONSPECIFIC SIGNS.
Other systemic problems may be seen with Brucella infection. Discospondylitis of the thoracic and/or lumbar vertebrae has been reported (Smeak et al, 1987; Kerwin et al, 1992). Endophthalmitis and recurrent uveitis have been associated with brucellosis (Gwin et al, 1980; Johnson and Walker, 1992). A low-grade nonsuppurative meningitis (without clinical signs) was observed in dogs that were experimentally infected. Dogs with brucellosis can have arthritis or polyarthritis as the only clinical signs (Forbes, 1990). Most dogs show no clinical signs, or owners complain of subtle signs such as a poor hair coat, listlessness, and exercise intolerance (Gordon et al, 1985).
ABORTION AND VAGINAL DISCHARGE.
The most common and obvious clinical sign of brucellosis in an otherwise healthy bitch is abortion between days 45 and 59 of gestation. The bitch may resorb fetuses and appear to be infertile because other clinical signs are absent. A bitch with brucellosis may abort puppies as early as day 30 of gestation or may carry pups nearly to term. A bitch with brucellosis also may deliver both living and dead fetuses. Surviving puppies are bacteremic for at least several months. Aborting bitches may lose two or three litters in succession (Nicoletti, 1989).
Aborted puppies usually appear to be partly autolyzed. The vaginal discharge present in some bitches aborting their litters may be brown or greenish gray (Purswell, 1992). This discharge usually contains large numbers of organisms, and extreme caution should be taken to prevent humans or dogs from coming into contact with it. Prolonged vaginal discharge after abortion is characteristic of this condition. In addition to the infected vaginal discharge, mammary secretions, urine, saliva, nasal secretions, and semen may contain organisms and may cause transmission (Carmichael and Greene, 1990).
Diagnosis of Brucella canis
NONSPECIFIC FINDINGS.
Hematologic and serum chemistry abnormalities in dogs with brucellosis are nonspecific, and the urinalysis results are usually unremarkable. Any inflammatory condition (involving the testes, intervertebral discs, joints, and the like) may be the result of brucellosis, but the disease is relatively uncommon. Even semen analyses and vaginal discharges from known diseased dogs show nonspecific changes, such as the head-to-head sperm agglutination with inflammatory cells described in the semen analyses from infected dogs (Greene and George, 1984).
SEROLOGIC TESTS
Background.
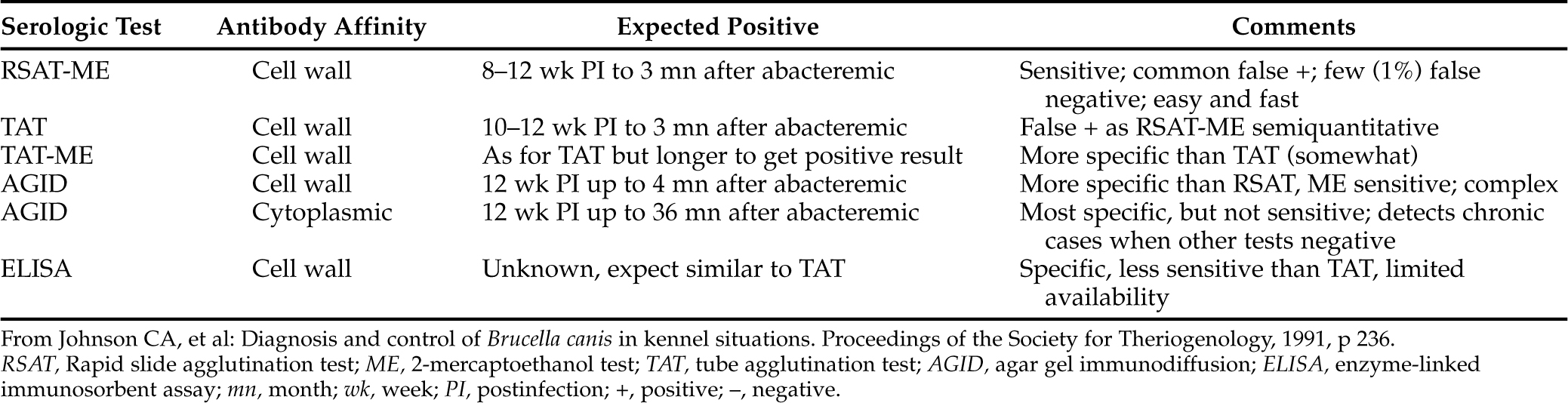
Serologic tests for the presence of antibodies to cell wall and cytoplasmic antigens of B. canis are considered reliable, but such titers may not be detectable for 8 to 12 weeks. When bacteremia subsides after 30 to 60 weeks of infection, the serologic titer results may decline or become equivocal (Johnson et al, 1991). Therefore serologic titers, like many tests, are not perfect. Titers may fluctuate even with persistent bacteremia, and titer magnitudes do not reflect the stage of disease. Declining titers may not correctly reflect success of therapy (Table 26-1). Thus serologic testing may provide presumptive, but not definitive, diagnosis of canine brucellosis.
Rapid Slide or Card Agglutination Tests (RSAT; RCAT).
The RSAT (Canine Brucellosis Diagnosis Test Kit, made by Pitman-Moore, Washington Crossing, NJ; a version is also made by Synbiotics, San Diego, CA) is a rapid in-hospital presumptive screening test that can be used by practitioners to accurately identify Brucella-negative dogs. Because the test is widely available and practical, it is used much more often than culture protocols. The serum used for the test should be free of hemolysis. B. ovis is used as antigen because of its similarity to B. canis. The test is performed by mixing the patient’s serum with a rose bengal–stained, heat-killed, B. ovis suspension on a glass slide. Agglutination of the bacteria is interpreted as suspicious for B. canis infection but is not by itself diagnostic (Greene and George, 1984).
The test is highly sensitive, and false-negative reactions are rare. However, when used according to the instructions, the kit identifies as many as 60% false-positive reactions. The false-positive results apparently are caused by cross-reactions between B. ovis antigen and antibodies to Bordetella bronchiseptica, Pseudomonas spp., a Moraxella-like organism, and other gram-negative bacteria (Greene and George, 1984). Therefore it may be stated with confidence that animals that do not show agglutination on the RSAT do not have brucellosis and that dogs that do show agglutination should be isolated and tested with the TAT.
A modification of the RSAT has been developed that uses 2-mercaptoethanol to reduce heterologous immunoglobulin M (IgM) agglutination (Badakhsh et al, 1982). This modification has been found to eliminate false-positive reactions (Nicoletti, 1989). However, the modified test could be falsely negative in the first few weeks after infection (Greene and George, 1984). An improved antigen was then described for the SAT; B. ovis antigen was replaced with B. canis (M–) cells, and the number of false-positive results declined (Carmichael and Joubert, 1987).
Tube Agglutination Test.
The test is performed by adding graded amounts of test serum to B. canis antigen solution to achieve different dilutions. The antigen solution is a suspension of heat-killed, washed B. canis organisms. A titer of 1:200 by the TAT is considered presumptive evidence of an active infection. Good correlations have been found between titers equal to or above 1:200 and recovery of the organism by blood cultures (Nicoletti and Chase, 1987b). A titer that is measurable but below 1:200 should be rechecked at least 2 weeks later.
The 2-mercaptoethanol tube agglutination test (2ME TAT) is similar to the TAT except for the addition of 2ME to the antigen solution. This is done in an attempt to increase test specificity. Obtaining positive titers with the TAT, after ME is added, may be delayed 1 to 2 weeks compared with the TAT, but there are fewer false-positive results (Greene and George, 1984; Nicoletti, 1989).
Agar Gel Immunodiffusion Test.
An AGID test using one or more antigens prepared by differing methods is available in some laboratories. The AGID test is recommended as an aid in confirming diagnoses suspected from RSAT, RCAT, or TAT results. The most specific but least sensitive AGID test used a protein antigen extracted from the cytoplasm of B. canis (Zoha and Carmichael, 1982). It was sensitive within 4 to 8 weeks of the onset of bacteremia and was positive for at least 12 months after the end of bacteremia, at times when other tests produced equivocal findings. The AGID test, however, may be negative in early infection when other tests would provide positive results (Nicoletti and Chase, 1987b). One advantage of the AGID test is the rapid seroconversion from positive to negative that may be detected when a dog is successfully treated.
The test is not widely available because AGID antigens are not readily prepared free from cross-reacting lipopolysaccharides. Furthermore, immunodiffusion procedures are generally limited to laboratories with specialized facilities and specifically trained personnel (Nicoletti, 1989). In 1993 the American Association for Veterinary Diagnostic Laboratories agreed that the Cornell University Diagnostic Laboratory would serve as a B. canis reference laboratory. Therefore serum samples from dogs that test positive on the RSAT, RCAT, or TAT should be sent to this laboratory for further testing. The Cornell diagnostic laboratory reevaluates the serum with the AGID test using cytoplasmic protein antigens that are more unique and specific to Brucella species than cell wall antigens (Johnston et al, 2001). However, the AGID test result may be positive in dogs that have been exposed to B. ovis, B. abortus, or B. suis because some of these cytoplasmic antigens appear to be shared among the Brucella species (Johnson and Walker, 1992).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree