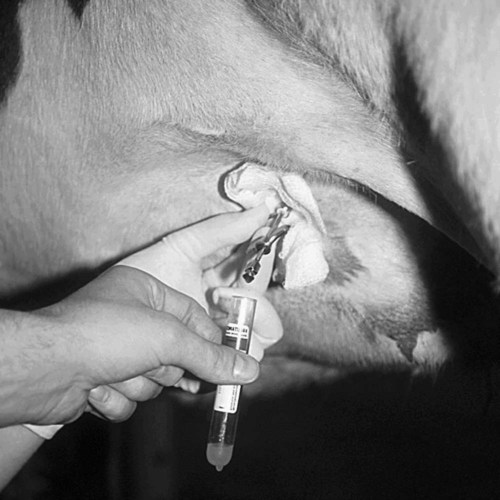
When you have completed this chapter, you will be able to • Understand the basic natural instincts of cattle and how they affect the handling and restraint of cattle • Set up and prepare the patient for each procedure, perform the procedure (when appropriate), or assist the clinician in performing diagnostic sampling and medication procedures • Properly insert and maintain an intravenous catheter and monitor the catheter for complications • Explain the rationale and indications for each of the clinical procedures described • Set up materials and equipment and prepare the patient as needed for the procedure • Perform or assist in necropsy and sample collection procedures and maintain a safe environment during these procedures To begin, the animal should be properly restrained (Fig. 11-1). Cattle tend to resist venipuncture, and it should never be attempted on an unrestrained animal. Simply placing a halter on the animal is not enough. The body must be restricted in movement, usually in a chute or head catch gate. Even when the animal is properly restrained, the technician should be prepared for a response by the animal when the needle penetrates the skin. The technician avoid standing or kneeling where he or she may be injured. After the site is cleaned, the vein is distended by placing all of the fingers or a fist firmly into the jugular groove. The diameter of the jugular vein is quite large (1–2 inches), and the vein cannot be completed occluded using just one finger or one thumb (Fig. 11-2). One should allow enough time for the vein to fill so that it can be readily seen. While one hand maintains distension, the other hand places a 16- or 18-gauge (ga), Because the diameter of the tail vein is considerably smaller than that of the jugular vein, needles larger than 18 gauge should not be used. An 18-, 19-, or 20-ga, 1- to The procedure is best performed in the proximal third of the tail, directly on the ventral midline of the tail. The coccygeal vertebrae in this area have hemal processes (arches), which are bony canals on the ventral aspect of the vertebral bodies. The hemal processes protect the coccygeal artery and vein, which run through the canal; therefore, venipuncture must be done between the vertebrae, where no hemal processes are present. The hemal processes are easily felt on the ventral midline as firm, bony protrusions. The soft space between any two hemal processes is palpated, and the needle is directed into the space at a 45- to 90-degree angle to the skin. The needle is advanced while aspirating gently on the syringe; the vessels are generally encountered The right and left milk veins course along the ventrolateral body wall of the thorax and abdomen. They provide major venous drainage of the udder, especially during lactation. They are easily identified as large-diameter tubular structures just beneath the skin, with a pronounced tortuous (twisty) course (Fig. 11-5). Numerous other texts describle blood analysis in great detail and should be used as references for complete analysis. Table 11-1 lists normal complete blood count values. Table 11-2 lists normal blood chemistry values for cattle. TABLE 11-1 Normal Complete Blood Count Values TABLE 11-2 From Aiello SE: The Merck veterinary manual, 8th ed. Whitehouse Station, NJ, Merck & Co., 1998 In cattle, females may be encouraged to urinate by “titillating,” which is a method of stimulating the perineal area (Fig. 11-7). The skin beneath the vulva is lightly stroked with the fingers or with straw until urination occurs. The tail should not be held during the procedure so as not to distract the cow. If this method does not work, repeated parting of the lips of the vulva may be effective. The initial urine stream is not collected because it contains more “contaminants” (debris and bacteria). A midstream sample is preferred and is collected into a clean container. If bacterial culture is to be performed, the container should be sterile. Urine stimulation in males is difficult. Manual stimulation of the prepuce is sometimes effective. A capped tube with threads is rubbed on the inside of the sheath to stimulate urination in males (Fig. 11-8). Fecal collection should be performed with a gloved hand. The technician should either take samples from the ground if not contaminated or lube the hand and obtain a sample from the rectum (Fig. 11-9A). To obtain a sample from the rectum, lubricate the gloved hand and place the fingers together so that all of the fingers and thumbs are touching. Gently insert the hand into the rectum. Never use extreme force to gain entrance to the rectum. It is not necessary to enter the rectum at great lengths; enter just enough to wipe the rectum wall with the fingers. Never separate the fingers. Just scope the rectum and remove the hand. It is common to turn the glove inside out and tie the top of the glove to keep the sample until return to the clinic or until the sample arrives at the laboratory (Fig. 11-9B).
Bovine Clinical Procedures
Diagnostic Sampling
Venous Blood Sampling

Jugular Vein
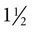 -inch needle through the skin and into the vein at a 45-degree angle to the skin surface. This should be done in one swift, committed motion, noting that bovine skin may be somewhat thicker than expected. The needle can be placed in either direction (cranially or caudally) for withdrawal of blood, depending on personal preference. Likewise, depending on preference, the needle can be placed alone and then the syringe attached when placement in the vein is confirmed, or both can be placed as a single unit.
-inch needle through the skin and into the vein at a 45-degree angle to the skin surface. This should be done in one swift, committed motion, noting that bovine skin may be somewhat thicker than expected. The needle can be placed in either direction (cranially or caudally) for withdrawal of blood, depending on personal preference. Likewise, depending on preference, the needle can be placed alone and then the syringe attached when placement in the vein is confirmed, or both can be placed as a single unit.

Coccygeal (Tail) Vein
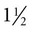 -inch needle is used. Usually the syringe is left attached during the procedure. Experienced operators may use Vacutainer tubes (Fig. 11-3).
-inch needle is used. Usually the syringe is left attached during the procedure. Experienced operators may use Vacutainer tubes (Fig. 11-3).
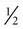 to 1 inch beneath the skin. If the needle contacts bone, slowly retract the needle and continue to aspirate. Once the needle bevel is in the lumen of the vein, maintain the position while withdrawing the blood sample (Fig. 11-4).
to 1 inch beneath the skin. If the needle contacts bone, slowly retract the needle and continue to aspirate. Once the needle bevel is in the lumen of the vein, maintain the position while withdrawing the blood sample (Fig. 11-4).
Subcutaneous Abdominal (Milk) Vein
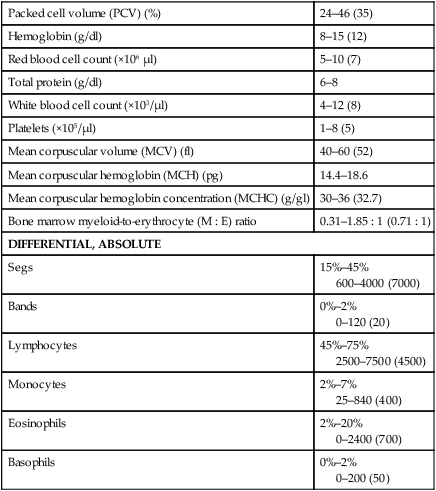
Packed cell volume (PCV) (%)
24–46 (35)
Hemoglobin (g/dl)
8–15 (12)
Red blood cell count (×106 µl)
5–10 (7)
Total protein (g/dl)
6–8
White blood cell count (×103/µl)
4–12 (8)
Platelets (×105/µl)
1–8 (5)
Mean corpuscular volume (MCV) (fl)
40–60 (52)
Mean corpuscular hemoglobin (MCH) (pg)
14.4–18.6
Mean corpuscular hemoglobin concentration (MCHC) (g/gl)
30–36 (32.7)
Bone marrow myeloid-to-erythrocyte (M : E) ratio
0.31–1.85 : 1 (0.71 : 1)
DIFFERENTIAL, ABSOLUTE
Segs
15%–45%
600–4000 (7000)
Bands
0%–2%
0–120 (20)
Lymphocytes
45%–75%
2500–7500 (4500)
Monocytes
2%–7%
25–840 (400)
Eosinophils
2%–20%
0–2400 (700)
Basophils
0%–2%
0–200 (50)
Blood urea nitrogen (mg/dl)
7.8–24.6
Creatinine (mg/dl)
0.6–1.8
Glucose (mg/dl)
42.1–74.5
Albumin (g/dl)
2.8–3.9
Total bilirubin (mg/dl)
0–0.8
Aspartate aminotransferase (AST)(µ/L)
45.3–110.2
γ-Glutamyltransferase (GGT) (µ/L)
4.9–25.7
Creatine kinase (µ/L)
14.4–107
Alkaline phosphatase (µ/L)
17.5–152.7
Lactate dehydrogenase (LDH) (µ/L)
308.6–938.1
Sodium (mEq/L)
134.5–148.1
Potassium (mEq/L)
4–5.8
Chloride (mEq/L)
95.7–108.6
Calcium (mg/dl)
8.4–11
Phosphorus (mg/dl)
4.3–7.8
Magnesium (mg/dl)
1.7–3
Urine Collection
Voided Urine Sampling


Fecal Collection
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Bovine Clinical Procedures

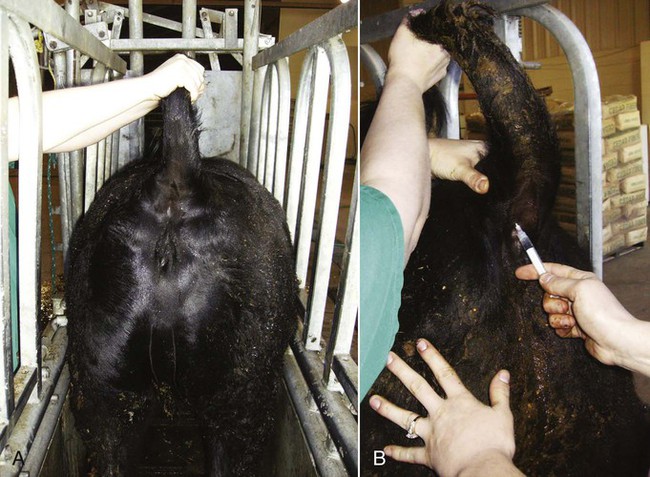

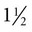 to 3 inches long must be used to penetrate the abdominal wall of cattle. Needle diameter may range from 18 to 20 gauge. The milk veins (subcutaneous abdominal veins) must be avoided (
to 3 inches long must be used to penetrate the abdominal wall of cattle. Needle diameter may range from 18 to 20 gauge. The milk veins (subcutaneous abdominal veins) must be avoided (