Chapter 8 Bone Marrow Examination
Sites for Bone Marrow Biopsy
Young animals have active (red) marrow throughout most skeletal bones. Active marrow recedes from long bones as adulthood is reached, because the bone marrow space expands faster than the blood volume as the animal grows.53 Once animals stop growing, blood cell numbers must be maintained, but increased production to accommodate growth is no longer required. As hematopoietic cells disappear, the marrow space is replaced by fat (yellow marrow) and is in a resting state. Hematopoietic cells may expand back into long bones if needed, as might occur in response to anemia. Active marrow remains in the flat bones (vertebrae, sternum, ribs, and pelvis) and proximal ends of the humerus and femur in adults.53
Ilium
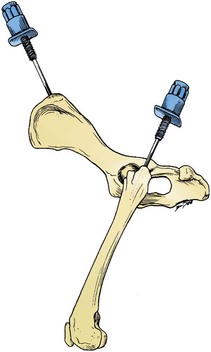
The iliac crest is often used as a site to aspirate/biopsy marrow in dogs and is sometimes used in cats.29,53 The biopsy needle is positioned so that it enters the greatest prominence of the iliac crest, parallel to the long axis of the wing of the ilium (Fig. 8-1). The wing of the ilium may also be aspirated at its central depression, which is caudal and ventral to the iliac crest. The tuber coxae has been used as a site for bone marrow collection in young horses, but adequate marrow samples cannot be obtained from this site in adult horses because of a lack of active marrow.58
Proximal Femur
For small cats and toy breeds of dogs, in which the ilium is especially thin, the marrow may be aspirated from the head of the proximal femur by way of the trochanteric fossa (see Fig. 8-1).29,53 This site is not suitable for obtaining a core biopsy.
Proximal Humerus
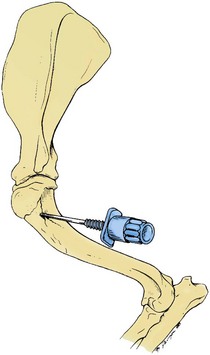
Aspiration of marrow from the cranial side of the proximal end of the humerus is also commonly done in small animals, especially in those that are obese.29 This site has also been recommended for use in calves.47 The greater tubercle is palpated, and the needle is inserted into the flat area on the craniolateral surface of the proximal humerus distal to the tubercle (Fig. 8-2).
Sternum
In large dogs, the third, fourth, or fifth sternebra can be aspirated/biopsied.53 The collection of marrow from the sternum poses the risk of inadvertent penetration of the thorax and damage to structures in the thoracic cavity. A short biopsy needle (preferably with an adjustable guard) should be used, and care should be taken to remain in the center of these bones to minimize the risk of pneumothorax, uncontrolled hemorrhage, or cardiac laceration. Although there is also some risk to the person collecting sternal marrow from standing large animals, the sternum (third or fourth sternebra) is the preferred site for collecting high-quality aspirates/biopsies from adult horses, cattle, sheep, and llamas. Sternebrae are identified by palpating ribs to their articulations with the sternum.47
Proximal Ribs
The proximal (dorsal) ends of the ribs have been used for bone marrow aspirates in large animals, although the bone is difficult to penetrate with biopsy needles in adults and can severely damage the needles.58,59 There is also a risk of pneumothorax or uncontrolled hemorrhage when ribs are biopsied.5
Technique of Bone Marrow Aspiration
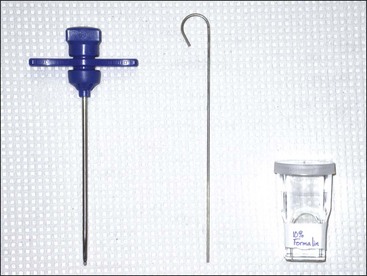
The needle used to aspirate marrow must have a removable stylet, which remains in place until the marrow cavity is entered to prevent obstruction of the needle’s lumen with cortical bone. A 16- or 18-gauge needle (Rosenthal, Illinois sternal, or Jamshidi) between 1 and 1.5 inches long is satisfactory (Fig. 8-3).
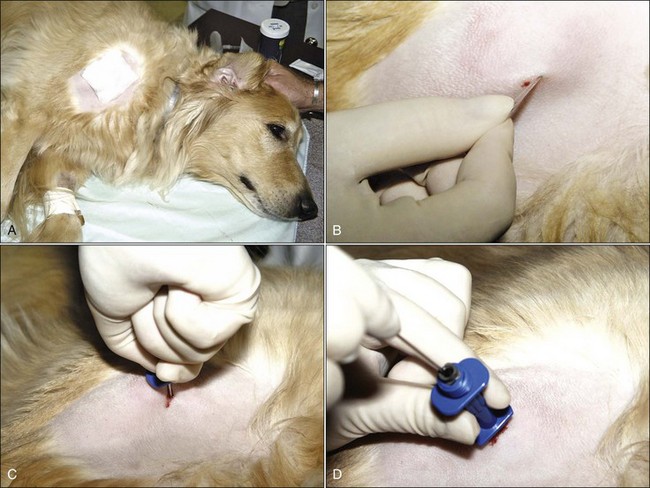
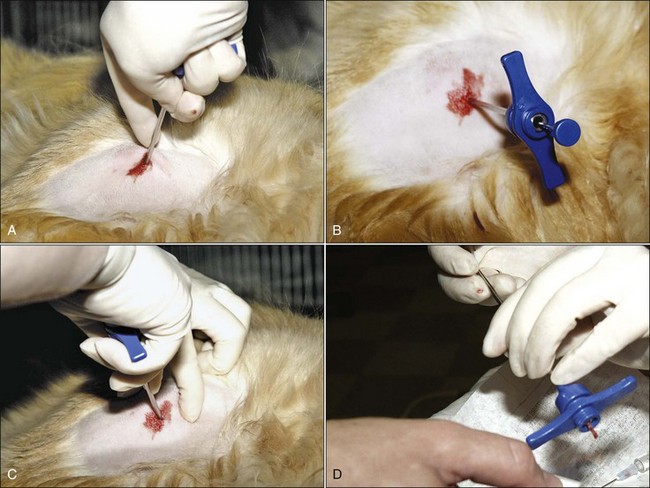
The usefulness of bone marrow aspirate cytology as a diagnostic aid depends on the proper collection of the bone marrow sample and preparation of high-quality marrow smears. In most cases with dogs and large animals, only local anesthesia is needed for aspiration biopsies. However, tranquilization is often used, especially in patients that resist positioning by manual restraint. Bone marrow aspirates are often done under light anesthesia in cats. Collection sites are prepared by clipping the hair and scrubbing the skin with antiseptic soap preparations (Fig. 8-4, A). A local anesthetic is injected under the skin and down to the periosteum overlying the site to be aspirated, and a small skin incision is made with a scalpel blade to facilitate passing the needle through the skin (Fig. 8-4, B). Sterile needles and gloves are always used, but the aspiration site is generally not draped. If general anesthesia is required for other procedures, bone marrow aspiration may be scheduled at the same time to minimize the stress on the animal.
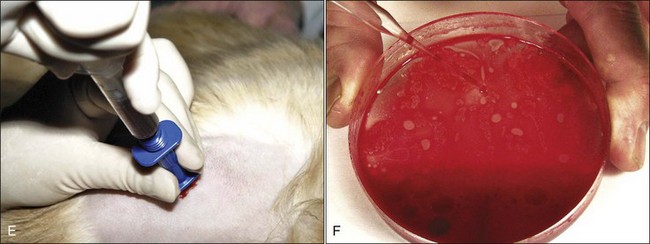
To enter the marrow space, moderate pressure is applied to the needle (with the stylet locked in place) as the needle is rotated in an alternating clockwise-counterclockwise motion (Fig. 8-4, C). Once the needle is firmly embedded into the bone, it is usually within the marrow cavity. The stylet is then removed (Fig. 8-4, D) and a 10- to 20-mL syringe is attached to the needle (Fig. 8-4, E). Vigorous negative pressure should be applied by rapidly pulling the plunger back as far as possible. If no anticoagulant is added to the syringe, the negative pressure is released, and the complete assembly is rapidly removed for smear preparation as soon as a few drops appear in the syringe. If marrow does not appear in the syringe, the stylet is replaced and the needle is repositioned for another aspiration attempt.
If no anticoagulant is used, smears must be prepared within seconds after bone marrow collection, because bone marrow clots rapidly. Smears prepared once clotting begins cannot be evaluated, because most of the nucleated cells will be lysed during smear preparation. We prefer to collect bone marrow into a syringe that contains EDTA as an anticoagulant, because it reduces the chance of clotting before a smear is prepared and allows time to prepare multiple smears that may be needed for special stains. Additionally, the ability to collect bone marrow particles in anticoagulated samples, prior to smear preparation, increases the likelihood of getting sufficient particles to make an accurate interpretation of the aspirate smear. An EDTA-containing solution can be prepared by adding 0.35 mL of sterile saline to a 7-mL EDTA tube, which yields a 2.5% or 3% EDTA solution, depending on whether the tube contains dry EDTA or EDTA dissolved in water. About 0.5 mL of this EDTA solution is drawn into the syringe using a regular needle prior to attaching the syringe to the bone marrow aspiration needle.30 Although smears need not be made immediately when collected with EDTA, they should be prepared within minutes after collection, because bone marrow cells (especially granulocytic cells) degenerate rapidly.
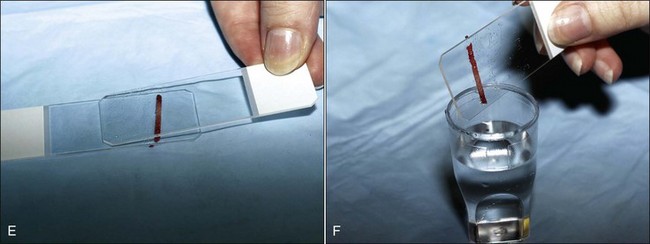
About 1 mL of marrow is aspirated into the syringe containing EDTA. Following mixing, the contents are expelled into a petri dish. It is important for accurate bone marrow evaluation that smears contain marrow particles (stroma and associated cells). Marrow particles appear as small white grains (flecks) in the blood-contaminated aspirate material. The particles tend to loosely adhere to the bottom of the petri dish when it is tilted. The particles are collected by pipette (Fig. 8-4, F) or a hematocrit capillary tube to use for smear preparation.
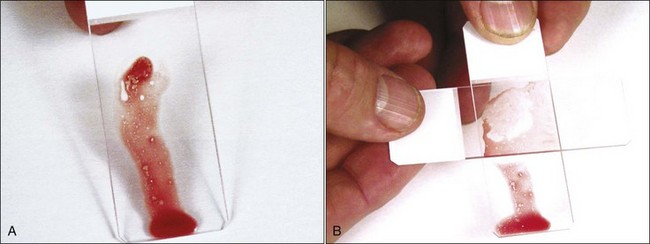
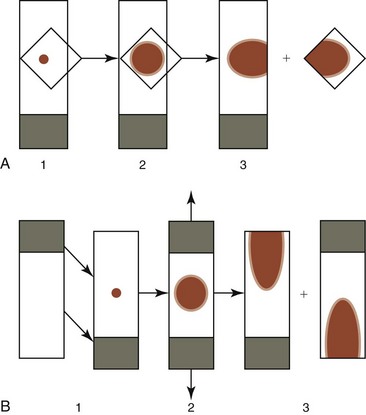
To concentrate particles in blood-contaminated aspirates, aspirated material can be placed on one end of a glass slide, which is then held vertically (Fig. 8-5, A). Particles tend to stick to the slide while blood runs off. A second glass slide is placed across the area of particle adherence, perpendicular to the first slide. After marrow spreads between the slides (Fig. 8-5, B), they are pulled apart in the horizontal plane.
Rather than use a perpendicular glass slide to spread the bone marrow particles, a square 22- by 22-mm 1.5-mm thick coverslip may be used in the same manner (Fig. 8-6, A). This produces both a coverslip and a glass slide preparation. Alternatively, one might consider using the two-coverslip method described for blood films in Chapter 2. The lighter weight of a top coverslip puts less pressure on particles than a top glass slide does. Consequently the use of coverslips may decrease the rupturing of nucleated cells, which happens more readily in samples collected with EDTA in saline compared with samples collected without an anticoagulant.30 If a marrow aspirate contains particles without excessive blood contamination, one can place the aspirated material in the middle of a slide and place a second slide on top of it; the slides are then slid apart horizontally, as shown in Figure 8-6, B.
If bone marrow is collected at necropsy, the marrow material should be mixed with a solution containing albumin before a smear is made to decrease cell lysis. One method recommends collecting bone marrow from an opened bone using a paintbrush and placing it on a piece of parafilm next to a drop of 22% bovine serum albumin solution. The paintbrush is then dipped in the albumin solution and mixed with the marrow material prior to smear preparation.47 Another method recommends mixing marrow collected from the cadaver with a 5% solution of bovine serum albumin (prepared in physiologic saline), followed by smear preparation.4
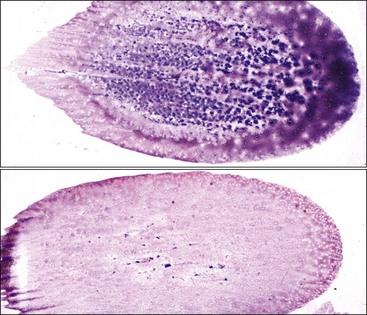
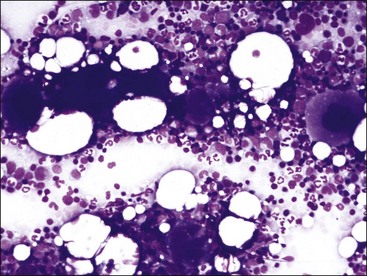
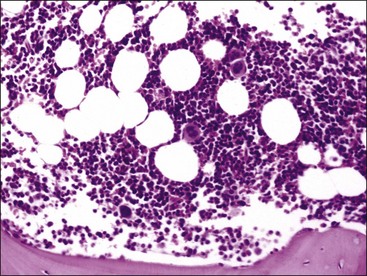
Smears are stained with a Romanowsky-type blood stain such as Wright, Giemsa, or a combination thereof. Satisfactory results can usually be obtained with the Diff-Quik stain, a rapid modified Wright stain. The appropriate staining time(s) for the stain or stains being used is determined with experience. Thicker smears will require longer staining times. About twice the time is required for staining bone marrow smears compared to blood films. Smears containing marrow particles will have blue-staining material on them, which is visible grossly (Fig. 8-7). When examined microscopically, particles contain blood cell precursors and stromal elements (Fig. 8-8). Fat is dissolved away during alcohol fixation, but it is represented in particles by the presence of variably sized unstained circular areas.
Technique of Bone Marrow Core Biopsy
Core biopsies are essential if there are repeated dry taps (e.g., failure to collect marrow particles by aspiration). Dry taps may be the result of technical error, but they can also occur when the marrow is packed with cells, as when a leukemia is present, and they usually occur when myelofibrosis is present. Dry taps or poor-quality samples are common when marrow aspirates are attempted on very young animals, even though the marrow is generally highly cellular. Core biopsy sections provide a more accurate way of evaluating marrow cellularity and examining for myelofibrosis, granulomatous diseases involving bone marrow, or metastatic neoplasia than do aspirate smears.12,55 Core biopsies are also needed to diagnose vascular abnormalities (edema and hemorrhage) and acute inflammation (fibrin deposition and focal neutrophilic infiltrates).74 To maximize information concerning bone marrow composition, we recommend collecting aspirate and core biopsy samples at the same time. Even when a core biopsy is not planned, it is advisable to have a core biopsy needle available in case a dry tap should occur.
Preparation of the animal and the site for a bone marrow core biopsy is the same as that described earlier for a marrow aspirate. Core biopsies require the use of special needles designed to cut a solid core of material. Jamshidi bone marrow biopsy needles, 11 to 13 gauge and 3 to 4 inches long, are used in our veterinary hospital (Fig. 8-9). Depending on the species and size of the animal, core biopsies may be taken from the wing of the ilium, head of the humerus, or sternum. Core biopsies and aspirates should be taken from distantly located sites to ensure that one collection does not result in disruption of the area where the other sample is being collected. Collection from two separate sites should also increase the likelihood of identifying tumor metastasis.12
With the stylet locked in place, moderate pressure is applied to the needle as it is rotated in an alternating clockwise-counterclockwise motion (Fig. 8-10, A). Once the needle is firmly embedded into the bone, the stylet is removed (Fig. 8-10, B) and the needle is advanced using the same clockwise-counterclockwise motion (Fig. 8-10, C). If possible, the needle should be advanced 1 inch to get sufficient material for evaluation. Once the needle has been advanced to its maximal depth, several 360-degree twists are made and it is withdrawn. The core within the needle is pushed out using a wire, which accompanies the needle. This is done by placing the wire in the tip of the needle and forcing the core out of the handle end of the needle (Fig. 8-10, D). Because the tip of the needle is tapered, pushing the core out through the tip would add crush artifacts to the core.
If attempts to aspirate bone marrow have resulted in dry taps or poor-quality smears, the core biopsy may be gently rolled between two glass slides (Fig. 8-10, E) or across a single glass slide using the tip of the needle and then stained in the same manner as aspirate smears. These roll preparations are generally of lower quality than aspirate smears. In particular, the number of megakaryocytes and amount of stainable iron present is generally underrepresented. After one or more roll preparations are made, the core is placed in fixative (Fig. 8-10, F) and submitted to a surgical pathology service, where it is decalcified, embedded, sectioned, stained with hematoxylin and eosin (H&E) and possibly other stains, and examined microscopically by a pathologist (Fig. 8-11). Fixatives other than formalin are sometimes preferred; consequently the surgical pathology service should be consulted prior to sample collection. Unfixed aspirate smears, core roll preparations, or other exfoliative cytology preparations should not be mailed in the same package with formalin-fixed tissue because the formalin vapors will interfere with the staining quality of cells in the exfoliative cytology preparations.
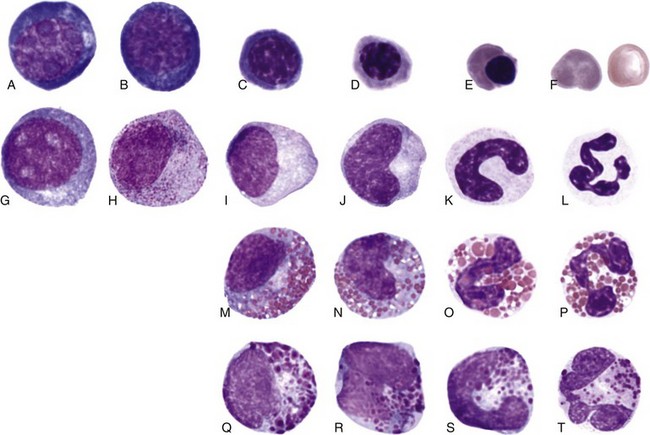
Morphologic Identification of Cells
This discussion focuses primarily on the morphologic appearance of cells in aspirate smears stained with Wright-Giemsa, but examples of core biopsy sections stained with H&E are also presented. Marrow particles appear as blue-staining areas in aspirate smears when viewed grossly (see Fig. 8-7). When examined microscopically, they contain blood cell precursors, vessels, reticular cells, macrophages, and plasma cells (see Fig. 8-8). Fat is dissolved away during alcohol fixation, but it is represented in particles by the presence of variably sized, unstained circular areas. Most particles in normal animals are composed of one-third to two-thirds cells (see Figs. 8-8, 8-11).
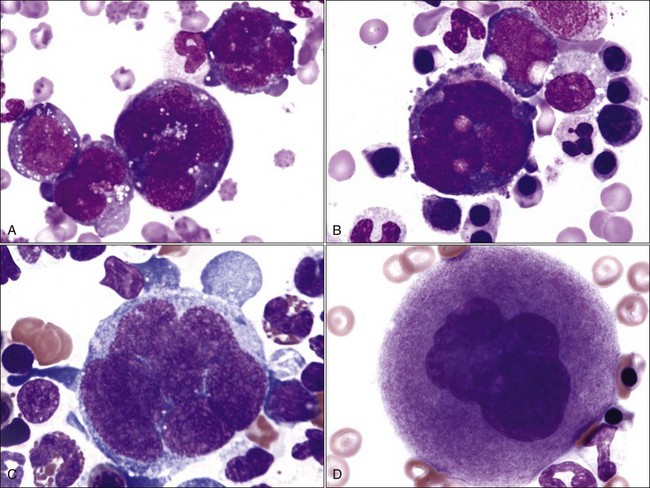
Megakaryocytic Series
Megakaryoblasts are the earliest recognizable cell in this series. They have a single nucleus and deeply basophilic cytoplasm (Fig. 8-12, A). This cell type is not recognized in most normal aspirate smears because it occurs in small numbers and is difficult to differentiate from other blast cells. Promegakaryocytes, which have two or four nuclei and deeply basophilic cytoplasm, are easily recognized (Fig. 8-12, A, B). These cells are much larger than leukocytes or nucleated erythroid precursor cells. Subsequent nuclear reduplications result in progressively larger basophilic megakaryocytes (Fig. 8-12, C). Nuclei in basophilic megakaryocytes are joined into a lobulated mass, making it difficult to count the number of nuclear reduplications that have occurred. The synthesis of magenta-staining cytoplasmic granules imparts a pink color to the cytoplasm characteristic of mature (granular) megakaryocytes (Fig. 8-12, D). Megakaryocytes are gigantic and vary from 50 to 200 µm in diameter, with larger cells having greater nuclear ploidy.
Erythrocytic Series
Morphologic changes that occur as cells of the erythroid series undergo maturation include diminution in size, decrease in nuclear-to-cytoplasmic (N : C) ratio, progressive nuclear condensation, and the appearance of red cytoplasmic color as hemoglobin is synthesized and accumulates within the cytoplasm (Fig. 8-13).
Rubriblasts
The earliest recognizable cell type in the erythroid series is the rubriblast. This is a relatively large cell with a high N : C ratio and intensely basophilic cytoplasm resulting from the presence of many polyribosomes. The nucleus of the rubriblast is usually almost perfectly round and the chromatin is finely granular, containing one or more pale blue to medium blue nucleoli (see Fig. 8-13, A).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree