Chapter 74 Bluetongue
Lessons from the European Outbreak 2006-2009
Cause and Epidemiology
Bluetongue (BT) is an insect-borne disease caused by the bluetongue virus (BTV), an Orbivirus in the family Reoviridae. BT viruses consist of 10 linear, double-stranded, RNA segments contained within three concentric structural protein shells. Variations in the proteins that make up the outermost shell, particularly the variable virus protein 2 (VP2), determine the virus serotype. 24 serotypes are currently recognized, with a 25th proposed.1,13
BT was first described in 1902 and was thought to be confined to Africa until reports of its spread to Israel in 1951, United States (1952), southern Europe (1956), Asia (1961), and Australia (1975).20 It is now endemic to the tropics and subtropics but recent sustained outbreaks outside this geographic range, such as those in northwest Europe (2006 to 2009), indicate that this pattern may be changing.9,11 Historically, incursions into Europe have been attributed either to movements of infected ruminants from the Maghreb to southern Europe or to infected vectors being blown to noninfected areas on the wind. Other sources include illegal importation of live attenuated BTV vaccines from the Republic of South Africa such as in the recent incursions of BTV6 and BTV11. The cause of the sudden appearance of BTV8 in Belgium in 2006 (a country in which BTV had never previously been recorded) remains unknown. Phylogenetic studies have indicated the likely origin to be sub-Saharan Africa, and its introduction could have been by any one of the aforementioned routes.6 A summary of the incursions of BTV serotypes into Europe since 1998 are shown in Figure 74-1.
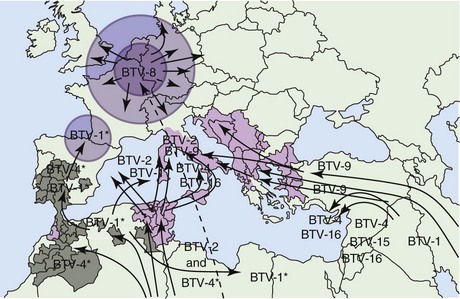
Figure 74-1 Spread of Bluetongue throughout Europe since 1998.
(Courtesy Institute of Animal Health, Pirbright Laboratory, Surrey, England.)
BTV relies on hematophagous female midges for its primary route of transmission among ruminant hosts. Less than 50 of the known 1500 Culicoides species have been shown to be competent BTV vectors; hence viral distribution is largely dependent on the occurrence of suitable climatologic factors for these particular midge species. In addition, temperature variations may also have a marked effect on transmission rates, even within the geographic ranges of these competent midges. After ingestion of a blood meal, the virus must infect and replicate within the insect’s midgut cells and again in their salivary gland cells prior to being inoculated into another ruminant. Only a small percentage of midges are susceptible to infection after viral ingestion via a blood meal; however, if reared at warmer temperatures, the infection rate and hence percentage of midges that may act as competent vectors increase. Warmer temperatures also lead to a reduction in the time required for the multiplication stage within the midge and increase the frequency of blood meals taken. At cool temperatures (<12° C), virus replication within the midge stops, and so does the midge’s ability to transmit virus.20
Until the outbreak of BT in northwest Europe, it was thought that Palaearctic midge species such as C. obsoletus and C. pulicaris, although shown to be competent in laboratory conditions, would not be able to sustain transmission of BTV in the field because of wide fluctuations in seasonal temperature. Experience gained during this outbreak has shown that for this newly introduced strain of BTV serotype 8 (BTV8), low-grade secondary routes of transmission (such as transplacental and oral) in combination with suspected latent infection in ruminants and midges, may sustain infection over the cold winter months at levels sufficient for BTV to be maintained at these latitudes.20 This newly apparent capability of BTV to maintain itself through temperate winters in conjunction with the highly temperature-dependent features of its epidemiology have led to a highly seasonal pattern of disease, with clinical cases in the northern European epizootics occurring almost exclusively between July and December each year.
Clinical Picture and Species Susceptibilities
It is generally accepted that all ruminant species and some camelids are likely to be capable of supporting BTV infection. Clinical expression of the disease is however highly variable and dependant on viral serotype, species, breed, immunologic status, environmental conditions and individual’s health status. Animals indigenous to endemic areas appear to be clinically resistant—however the mechanism of their resistance is unknown and could be either attributed to natural or acquired immunity.19
The pathology and pathogenesis of the disease has been reviewed in detail by Maclachlan and colleagues11 and the clinical picture seen in domestic species is well documented. The classic clinical signs of fever, nasal discharge, dyspnea, cyanosis of the tongue, oral lesions and ulcers, edema of the head and neck, lameness, and hyperemia of the coronary band are caused by virus-mediated vascular injury. These signs are most frequently seen in sheep, in which mortality rates may be 30% or higher. Cattle rarely show clinical disease, with the exception of the European BTV8 strain.20
Little has been published about the clinical susceptibility of nondomestic species. In North America, white-tailed deer (Odocoileus virginianus), prong-horn antelope (Antilocapra americana), and desert bighorn sheep (Ovis canadensis) are known to develop severe disease similar to that described in domestic sheep.19 Infection is not confined to ruminants and camelids but is also occasionally seen in carnivores. Abortion and death has been reported in dogs injected with a BT-contaminated vaccine and in a European lynx fed infected meat6,10,11 and natural asymptomatic infection is known to occur in endemic African carnivores. The incursion of BTV8 into Europe has enabled European zoos to provide a unique contribution to our knowledge of species susceptibility as they hold immunologically naïve individuals representing a wide taxonomic and geographic spectrum.
A survey of all 313 European Association of Zoos and Aquariams (EAZA) zoos was undertaken in January 2008 to collate data on clinical disease seen during the 2007 BTV season. Of these, 49 zoos had confirmed BTV8 cases within 20 km and could be classified as at risk of infection. These 49 zoos held over 1000 susceptible individuals of 53 different species and seven ruminant families indigenous to Europe, North and South America, Africa, and Asia. Clinical disease was seen in 62 individuals (6% of the at-risk population), spread among 13 zoos (27% of at-risk collections).17
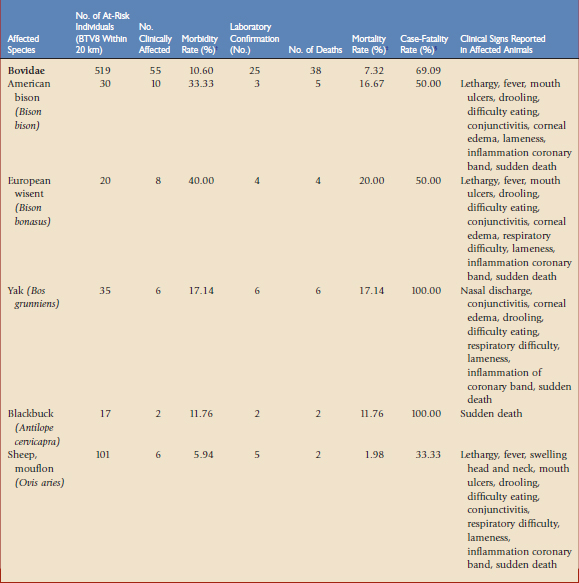
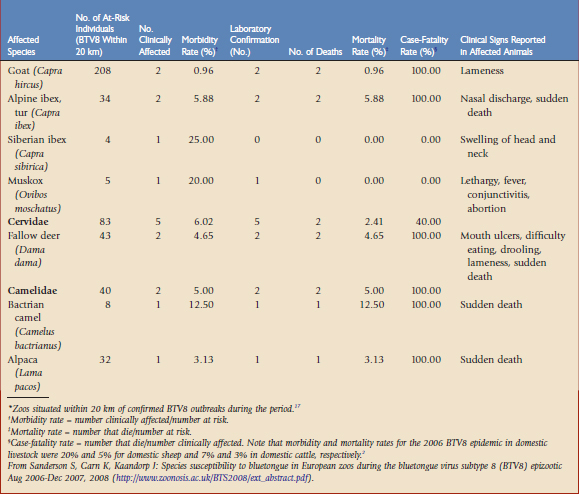
Mortality and morbidity rates and the clinical picture seen in each affected species are summarized in Table 74-1. The subfamilies bovinae and caprinae are the most susceptible of the ruminants to clinical disease, with four species showing morbidity rates higher than 20% and mortality rates higher than 10%. The average case-fatality rate for the affected bovinae and caprinae species was 69%. All the affected ruminant species in this study were indigenous to Europe, Asia, or South America. Clinical signs in these species are consistent with those recorded for BTV8 infection in domestic livestock and with those reported in yak.2,12 It is noteworthy that despite over 200 African ruminants of 20 species being held by zoos in at-risk areas, none of these were reported to have shown clinical signs of infection. This is consistent with observations in Africa that indigenous antelope do not develop clinical disease.19
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree