Fig. 5.1
Ewe with hyperaemic muzzle and shallow erosions and crusts on the nostril
Nasal discharge, which became later sometimes mucopurulent, and resulting dyspnoea could often be observed.
Foot lesions developing with the subsidence of fever represented a frequently detected disease manifestation. The coronary band is hyperaemic often combined with petechial haemorrhages.
An investigation in sheep farms in 2007 revealed that stillborn lambs showed clinical signs such as crusts and lesions of the oral mucosa (Fig. 5.2a). One of the farmers reported that the newborn lambs were physically and motorically retarded for several weeks. Feet lesions were apparently so painful that some of the sheep were reluctant to walk for weeks even after the acute symptoms had healed. Instead, they crawled on their carpal joints (Fig. 5.2b). When the animals were supported or when they got shooed, they walked on their feet for a little while; however, they immediately fell back to their previous behaviour when they were left alone.
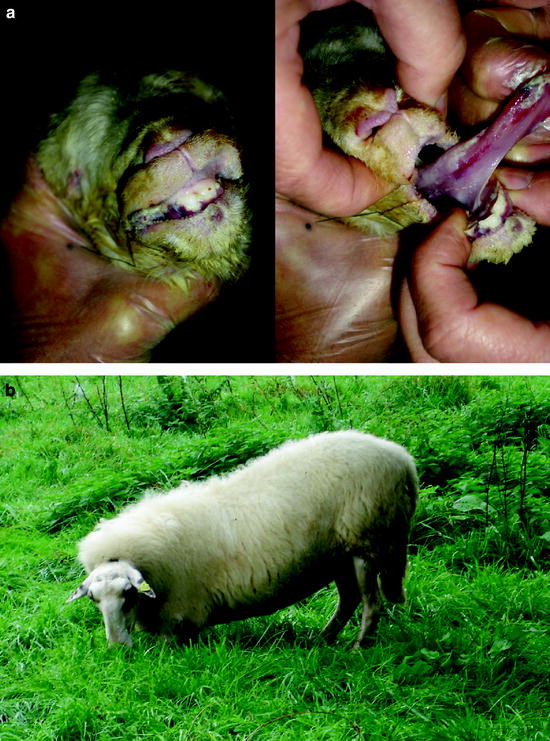

Fig. 5.2
Clinical signs of BT in sheep; (a) lips and tongue of a stillborn lamb with crusts and lesions in the oral mucosa; (b) sheep walking on carpal joints
5.3.2 Goats
Reports on clinical signs with BTV-8 virus strains in goats are rare. During experimental studies with BTV-8 in Dutch dairy goats, fever, signs of general illness, apathy, dysphagia, diarrhoea and lameness were observed (Backx et al. 2007). The discrepancy between the observations in the field and in laboratory experiments might be explained by different routes of infection, e.g. intravenous injection vs. the natural route of infection via biting midges or by vector preferences or the types of husbandry systems (Backx et al. 2007). During the BTV-8 epidemic in Germany in 2006–2009, a total of 26,954 BTV-8-infected premises were recorded (TSN database; 15.11.2011, 1135 hours), among which there were 132 holdings where goats were reported as clinically affected (TSN database; 15.11.2011, 1135 hours). This shows that goats were affected by this epidemic in Germany, but only to a limited extent.
5.3.3 Cattle
It is thought that cattle have now largely replaced antelopes as a maintenance host of the virus in Africa (Gerdes 2004). However, before the BTV-8 epidemic in northern Europe, natural and experimental BTV infection of cattle was considered asymptomatic in the vast majority of cases (Maclachlan et al. 1992; Maclachlan 2011). A transient febrile response, lacrimation and salivation were occasionally observed in infected animals (Erasmus 1990).
At the beginning of the BTV-8 epidemic in Central Europe, clinical symptoms such as fever (40–41°C) for 2–14 days, severe nasal discharge, lacrimation, facial oedema and nasal excoriations were described in adult cattle (Mehlhorn et al. 2007). However, using a field virus strain that originated from a BT outbreak in the Netherlands in an experimental infection, no pyrexia was recorded in any of the cattle (four 6-month-old male Holstein-Friesian calves) at any stage of the experiment (Darpel et al. 2007). One of the first German BTV-8 outbreaks was detected in a beef suckler herd (57 cattle, Limousin breed) in North Rhine-Westphalia. One cow showed a bilateral conjunctivitis, oedema of the eyelids combined with petechial haemorrhages on the swollen mucous membranes of the eyelids. Additionally, strong lacrimation was obvious as a predominant clinical sign (Fig. 5.3). The local tissue damage was complicated by bacterial infection.
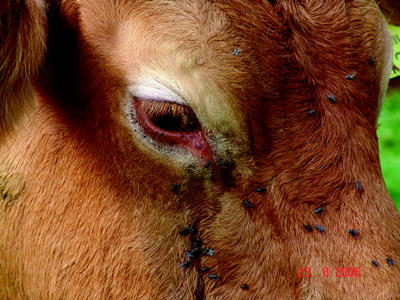

Fig. 5.3
Limousin cow with swollen eyelids, lacrimation and petechial haemorrhages
Ulcers and erosions may occur in the oral mucosa (Fig. 5.4). The skin of the muzzle was inflamed at the beginning of the disease; later, cracks and peels could be observed (Fig. 5.5).
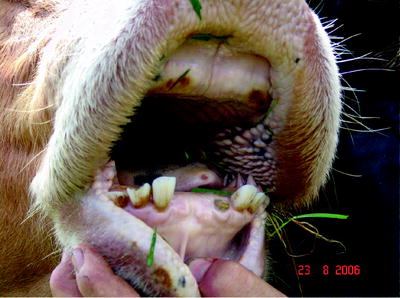
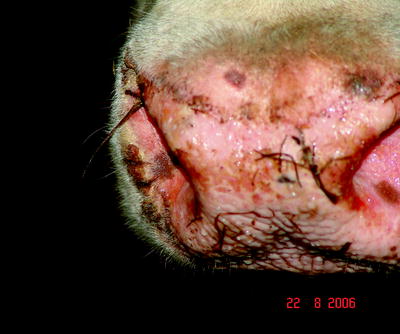

Fig. 5.4
Erosion on the oral mucosa in healing after BTV-8 infection

Fig. 5.5
Erosions, hemorrhagic lesions accompanied by bacterial superinfections and inflammatory foci of on the muzzle of a BTV-infected cattle
Large-scale erosions or haemorrhagic lesions in the skin of the teats were quite often reported in dairy cattle (Fig. 5.6). These lesions sometimes resulted in detachments of bigger parts of the skin of the affected teats. This caused pain during milking or nursing. Later on, these lesions were also affected by bacterial infection or characterized by the formation of crusts.


Fig. 5.6
Large-scale hemorrhagic lesions on the teats of BTV-8 infected cattle
Lesions involving coronitis and inflammation of the whole foot region were also frequently reported. The coronary bands were hyperaemic, swollen and inflamed. Therefore, stiffness or lameness is common in BTV-infected cattle (Erasmus 1990).
5.4 Diagnosis
5.4.1 BTV Isolation
For the direct detection of bluetongue virus, antigen or genome, whole blood is preferred over plasma because BTV is closely associated with red blood cells (Brewer and Maclachlan 1992; Nunamaker et al. 1992). Recommended specimens for the isolation of bluetongue virus are blood of live animals or spleen samples collected at necropsy. Heparinized or EDTA-treated blood that has been washed several times with phosphate-buffered saline to remove BTV antibodies is best suited for virus isolation. The release of virus particles from the erythrocyte membrane by sonication after washing can increase the success of the isolation. BTV-positive spleen samples are homogenized in cell culture medium and cleared by centrifugation. The purified supernatants can then be used for the inoculation of cultured cells or embryonated chicken eggs. For the latter, most laboratories use the intravenous inoculation method published by Goldsmit and Barzilai (1985). BTV-infected chicken embryos usually die within 2 and 7 days, and appear cherry red as a result of massive haemorrhages.
Besides mammalian cell lines (e.g. baby hamster kidney cells [BHK-21], African green monkey kidney cells [Vero] or bovine aorta endothelial cells), several insect-derived cell lines (e.g. Aedes albopictus clone C6/36 or Culicoides variipennis larval [KC] cells) can be used for isolation and propagation of BTV (Clavijo et al. 2000). The passage of virus in cell culture typically results in an adaptation of the virus to the in vitro conditions (Gould et al. 1989).
A highly sensitive and reliable method for the isolation of BTV is the inoculation of susceptible animals, mainly sheep or cattle. Although animal experiments are very expensive and ethical considerations have to be taken into account, an important advantage can justify this approach. Based on the large volume of inocula (up to 500 ml sample material are tolerated during intravenous injection), the sensitivity is very high. For samples with borderline infectivity (blood from animals in a late stage of infection or semen samples with a low viral load), the inoculation of susceptible animals can be the only way to propagate the virus (Hourrigan and Klingsporn 1975; Eschbaumer et al. 2010a, b).
Laboratory animals such as mice are not regularly used for BTV diagnosis and research. Nevertheless, the intracerebral inoculation of BTV isolates in 2–3 days old suckling mice causes clinical signs and death within 2–5 days after inoculation. Recently, a novel mouse model for BTV using type I interferon-receptor-deficient (IFNAR−/−) mice was developed (Calvo-Pinilla et al. 2009b; Eschbaumer et al. 2010b). Owing to the high susceptibility of these mice to fatal infection with BTV, they can also be used for virus isolation from samples harbouring a small viral load.
5.4.2 Molecular Diagnosis
Historically, confirmation and classification of BTV isolates have been based on immunological assays such as the indirect immune fluorescence test (Ruckerbauer et al. 1967; Jochim and Jones 1983), the complement fixation test (Shone et al. 1956), the haemagglutination assay (van der Walt 1980; Hübschle 1980), electron microscopy (Gould et al. 1989; Nunamaker et al. 1992), virus neutralization assays (Howell 1960) and competitive antigen-capture ELISAs (Mecham 1993; Mecham and Wilson 2004). With the development of nucleic acid detection methods in the late 1980s, cloned cDNA segments of several BTV serotypes were used to define the genetic relationships between and within the BTV serotypes (Unger et al. 1988; Ritter and Roy 1988). The introduction of the polymerase chain reaction (PCR) in the beginning of the 1990s revolutionized the molecular diagnosis of BTV (Wade-Evans et al. 1990; McColl and Gould 1991). In the following years, several improvements of BTV genome detection by PCR were published. Besides one-step RT-PCR assays, nested PCR systems and multiplex PCR systems for the identification of different BTV serotypes circulating in one region were developed (Wilson and Chase 1993; Katz et al. 1994; Aradaib et al. 1998; Zientara et al. 2004). Primers of different segments were used for the amplification and characterization of BTV strains (see Hoffmann et al. 2009a, for a review). For group-specific assays, conserved regions of the segments 5, 6, 7 and 10 were identified (Aradaib et al. 1998; Pierce et al. 1998; Bandyopadhyay et al. 1998; Anthony et al. 2007). Specific primers for the VP2 gene were developed and used in singleplex or multiplex assays to determine the BTV serotype (Wilson and Chase 1993; Eschbaumer et al. 2011b).
A new era for the molecular diagnosis of BTV began with the development of the real-time RT-PCR technology in the 1990s (Higuchi et al. 1993; Wittwer et al. 1997). Prior to the BTV-8 outbreak in Europe in the summer of 2006, only very few real-time quantitative RT-PCR (RT-qPCR) assays for the detection of BTV had been published. The first used primers were designed for the detection of the NS1 gene (Seg-5) (Wilson et al. 2004). However, this assay detected only 11 out of the 19 serotypes tested. The same year, another RT-qPCR was published using Förster resonance energy transfer (FRET) probe technology targeting genome segment 2 (VP2) (Orru et al. 2004). In 2006, an RT-qPCR assay was developed using a conserved region in RNA segment 5 of BTV-2 and BTV-4 (Jimenez-Clavero et al. 2006). This assay detected all of the recent Mediterranean isolates that were tested, BTV vaccine strains for serotypes 2 and 4 as well as 15 out of the 24 BTV reference strains. In the European outbreak of BTV-8, however, this assay showed a reduced sensitivity for the field strain of BTV-8 compared to other assays (Batten et al. 2008a). In the same year, an RT-qPCR was developed using a molecular beacon (MB) fluorescent probe designed within the NS3 conserved region of segment 10 (Orru et al. 2006).
Since the start of the northern European outbreak in August 2006, many RT-qPCR assays have been developed. Most of these were assays for the detection of all 24 serotypes identified at this time (Toussaint et al. 2007; Shaw et al. 2007), using conserved regions of the VP1 (seg-1) and NS1 (seg-5) genes. Nevertheless, the reduced sensitivity for the novel BTV serotypes 25 and 26 (Hofmann et al. 2008; Maan et al. 2011a, b) confirms the necessity for regular verification of the functionality of such pan-BTV assays. The parallel use of independent assays with equivalent diagnostic sensitivity and specificity can overcome the limitations of one assay. Another option is the application of pan-orbivirus assays, which use primer binding sites in the polymerase gene (seg-1) that are conserved among all currently known orbiviruses (Palacios et al. 2011). In addition to the group-specific (pan-BTV) real-time RT-PCR assays, serotype-specific BTV assays were developed and validated by diagnostic laboratories (Mertens et al. 2007; Hoffmann et al. 2009a, b). Furthermore, the advantages of the real-time PCR technology can be used for high-throughput analyses (Vandemeulebroucke et al. 2010). The use of robotics for automated extraction of BTV RNA combined with the co-amplification of the BTV target and an internal control RNA ensures a high diagnostic reliability during the investigation of large sample batches (Toussaint et al. 2007; Vandenbussche et al. 2010). These can be blood and tissue specimens in outbreak situations or insect samples from entomological monitoring programmes. High-throughput RNA extraction and real-time RT-PCR are particularly indispensable for the BTV analysis of extensive numbers of midges (Hoffmann et al. 2009c; Vanbinst et al. 2009).
5.4.3 Serology
Historically, BTV antibody detection in serum relied on complement fixation and agar gel immunodiffusion. Both, however, proved inferior to enzyme-linked immunosorbent assays (ELISAs) and were eventually replaced (Afshar 1994; Hamblin 2004). Highly sensitive ELISAs can pick up the humoral immune response to BTV as early as 1 week after infection (Batten et al. 2008a; Oura et al. 2009). Several systems are commercially available, mostly detecting antibodies against VP7, a BTV structural protein that is largely conserved across all serotypes. Three competitive ELISA kits (by ID VET, IDEXX and VMRD) are currently licensed for use in Germany. A latex agglutination test (Yang et al. 2010a) and immunochromatographic strips (Yang et al. 2010b) have recently been proposed, promising faster sample turnover and the possibility of pen-side testing.
Apart from the competitive ELISAs, an indirect assay is available for individual and bulk milk samples (Kramps et al. 2008; Chaignat et al. 2010; Mars et al. 2010). Double-antigen sandwich ELISAs, which use peroxidase-labelled antigen to detect captured antibody (Laman et al. 1991), display superior sensitivity early in infection and more reliably detect vaccine-induced antibody (Eschbaumer et al. 2009; Oura et al. 2009). Two kits (by ID VET and Prionics) are commercially available in Germany.1 However, their bias for multimeric antibody molecules such as immunoglobulin (Ig) M may have a negative impact on sensitivity during the transition from IgM to IgG in the development of the immune response (Eschbaumer et al. 2011a).
Beyond the performance of a group-specific assay, serotyping usually requires labour-intensive neutralization tests against a panel of reference viruses (Hamblin 2004). A serotype-specific antibody ELISA for BTV-8 has recently been implemented, but data on its performance are not yet available.
Regardless of the test format, the widespread use of inactivated whole virus vaccines in Europe (Zientara et al. 2010) interferes with serological surveillance. The commercially available VP7-based tests are unable to differentiate between infected and vaccinated animals (Mertens et al. 2009). Theoretically, inactivated vaccines should only elicit antibody responses to structural proteins. The discrimination potential of ELISAs based on non-structural proteins NS1 or NS3 has been demonstrated (Anderson et al. 1993; Barros et al. 2009) but is highly dependent on the purity of the vaccine. The carryover of non-structural proteins from the culture system used to produce the vaccine may result in antibodies to those proteins in vaccinated animals (Alpar et al. 2009), particularly after repeated vaccinations. Recent attempts at establishing a commercial NS1 ELISA were not successful. Vaccination with inactivated vaccines from different companies led to an increased number of unspecific results in the test, which, consequently, was not released by the manufacturer.
5.5 Epidemiology
5.5.1 Disease Transmission
When BTV serotype 8 (BTV-8) first appeared in Central Europe, no data on the putative vectors were available. Transmission of BTV had generally been supposed to be associated with Culicoides imicola, the most efficient and widespread vector in the Old World (Meiswinkel et al. 2007). Since this species is restricted to Africa and southern Europe, the occurrence of BT in more northern countries and its effective transmission by indigenous vectors was surprising, despite evidence from Italy suggesting that also C. pulicaris and midges of the C. obsoletus group can harbour BTV (Caracappa et al. 2003; Savini et al. 2004).
Meanwhile, entomological monitoring has shown that C. imicola is still not present in Germany and that members of the C. obsoletus and C. pulicaris complexes are relevant vectors for BT in Germany and Central Europe (Hoffmann et al. 2009c; Mehlhorn et al. 2009a). They are small midges of approximately 1–2 mm length which tend to be active in the evening, night and dawn. The length of time between ingestion of virus by a midge and its appearance in the saliva, the so-called extrinsic incubation period, is influenced by both temperature and salivary proteases of the vectors. At temperatures of 10–30°C, the extrinsic incubation period becomes progressively shorter with increasing temperatures since:
Midges feed more frequently.
Both the reproductive cycle of midges and virus replication in midges are faster.
A greater proportion of the midge population becomes vector competent.
Possibly more midge species become vector competent (Hateley 2009).
In Germany, transmission of BT is apparently interrupted or at least significantly reduced during the cold season (late autumn, winter and early spring) as a consequence of the low temperatures, which reduce the activity of Culicoides biting midges and BTV replication in the midgut of the biting midges. However, BTV might persist during the winter either in the vector population or in the host population (Wilson et al. 2008). Another possible way of overwintering is persistence in an as yet unknown wild ruminant population, e.g. red deer. In Belgium, relatively high levels of seroprevalence have been observed in red deer during 2007 and 2008. By contrast, the seroprevalence in roe deer was low, which was explained by the fact that red deer live in large groups, move more and therefore might be more exposed to insects (Linden et al. 2010). The results of the German wildlife monitoring on BT provide no evidence for the conclusion that a reservoir for BTV might have formed in wild ruminants in Central Europe. However, the relative importance of the remaining potential overwintering mechanisms remains unclear, too (Napp et al. 2011). As active midges were also found in the cold season, although in reduced numbers and mostly close to or within stables, a true midge-free period does not exist in Germany (Mehlhorn et al. 2009b).
Several investigation programmes were set up to share European BT outbreak data, e.g. in the BT51 group and as part of EPIZONE, an EU-funded Network of Excellence. Special attention has been paid to both mechanistic and stochastic predictive modelling which included a wide range of predictor variables to assess the spread of BTV (Faes et al. 2011; de Koeijer et al. 2011; Ducheyne et al. 2011; Willgert et al. 2011).
5.5.2 Introduction of Bluetongue Disease into Germany
In 2006, the first infections with bluetongue virus of serotype 8 (BTV-8), which was initially restricted to the sub-Saharan region, Asia and South America, invaded Central Europe. On August 18th, BTV-8 was confirmed in the Netherlands (EU Rapid Press release Nr. IP/06/1112, ProMedMail, 20060828.2448) and Belgium (Toussaint et al. 2007). On 21 August 2006, the first outbreak was reported in the neighbouring district of Aachen on the German side of the border (OIE, immediate notification, Conraths et al. 2007). The virus hit an area with a high density of BT-naive animals, presence of suitable vectors (Culicoides spp.), climatic conditions favourable for virogenesis in local biting midges and for transmission. So far, the epidemiological situation in Germany has been dominated by BTV-8 (Conraths et al. 2009; Gethmann et al. 2010), but a few cases of infections with BTV-6 and a single case of BTV-1 in an imported animal were reported (Eschbaumer et al. 2010a).
In addition to entomological patterns, much research has been devoted to the determination of the most likely time and place of introduction of BTV-8 (Saegerman et al. 2010). The following hypotheses to explain the introduction of BTV-8 to Western Europe were taken into consideration:
Legal or illegal import of viraemic susceptible animal species.
Legal or illegal import of infected non-susceptible animal species, especially against the background that the World Equestrian Games took place in Aachen at the time when the first BT cases were noticed.
Introduction via infected vectors, especially because midges are so light that they can drift by wind over hundreds of kilometres (Ducheyne et al. 2007). Alba et al. (2004) confirmed the possibility of introduction of infected midges to the Balearic Islands from Sardinia during the BT outbreak in the year 2000. It has also been proposed that BTV-infected biting midges might have been moved from northern Africa to Spain, Portugal or Italy by wind across the Strait of Gibraltar and the Mediterranean Sea in 2006 (Gloster et al. 2007; Hendrickx 2008). Another alternative is that infected vectors might have been directly introduced to Europe, e.g. by airplane with flowers imported from regions where BTV is enzootic. This hypothesis was also considered when BTV-6 occurred in the district of Grafschaft Bentheim (Lower Saxony) on the border to the Netherlands, although the respective virus closely resembled an isolate used in an attenuated live vaccine (Eschbaumer et al. 2010a).
Despite intensive epidemiological investigations, the source of the introduction has never been unambiguously identified.
5.5.3 Course of the 2006–2009 Bluetongue Epidemic in Germany
Until the end of 2006, a total of 890 cases/outbreaks were reported from the German federal states of North Rhine-Westphalia, Hesse, Rhineland-Palatinate and Lower Saxony to the German Animal Disease Notification System (TierseuchenNachrichtenSystem, TSN; accessed 23/11/2011). Between January and April 2007, when no transmission was expected, 185 further outbreaks were reported (Table 5.1). It is likely that at least the vast majority of infected animals detected in winter 2006/2007 had contracted BT in summer or autumn 2006.
Table 5.1
Number of reported outbreaks 2006/2007 and affected species
Year | Month | Cattle | Sheep | Goats | Wildlife | Total |
|---|---|---|---|---|---|---|
2006 | August | 35 | 4 | – | 1 | 40 |
2006 | September | 64 | 37 | – | – | 101 |
2006 | October | 295 | 170 | – | 4 | 469 |
2006 | November | 137 | 95 | – | 5 | 237 |
2006 | December | 37 | 3 | – | 3 | 43 |
2007 | January | 80 | – | – | 1 | 81 |
2007 | February | 52 | – | – | – | 52 |
2007 | March | 19 | 1 | – | – | 20 |
2007 | April | 31 | 1 | – | – | 32 |
Total | 750 | 311 | 0 | 14 | 1,075 | |
By analysing the outbreak data as reported to the TSN database and comparing them to the number of animals kept on the affected farms, it became apparent that at least 67,080 cattle, 9,825 sheep and 56 goats were present on premises affected by BTV-8 between August 2006 and April 2007 (Table 5.3). Of these animals, 1,529 cattle (2.28%) and 592 sheep (6.03%) were found infected. Eighty-four cattle and 222 sheep died. The case-fatality rate was much higher in sheep (37.5%) than in cattle (5.5%). These calculations are based on the assumption that all BT cases were reported. Since the infections caused only mild disease or remained even clinically inapparent in some animals, in particular cattle, it is likely that there was a substantial level of underreporting. As a consequence, the case-fatality rate in cattle might be slightly overestimated (Conraths et al. 2009).
Apparently, BTV-8 overwintered in the region and flared up again in 2007 to spread over most of western Germany during summer and autumn 2007. The first outbreak after the end of the cold season was confirmed in June 2007 in the district Oberbergischer Kreis, North Rhine-Westphalia, when a sentinel animal (cattle) sampled in May 2007 tested positive (Hoffmann et al. 2008).
The infection also re-emerged in other European countries that had been affected in 2006. BT spread rapidly through Belgium, the Netherlands, Germany, France and Luxembourg and reached the Czech Republic, Denmark, Italy, Spain, Switzerland and the United Kingdom.
Between May 2007 and April 2008, more than 22,600 cases/outbreaks were reported from Germany (Table 5.2; Conraths et al. 2009; Gethmann et al. 2010). Due to the enlargement of the affected territory in 2007, the exposed population of animals kept in farms with BT cases rose to at least 1,501,994 cattle, 505,934 sheep and 3,736 goats. The number of diseased animals on these farms amounted to 33,839 cattle, 32,158 sheep and 227 goats (Table 5.3). While mortality remained at relatively low levels as in 2006, the case-fatality rate rose to 10.8% in cattle and 41.5% in sheep.
Table 5.2
Number of reported outbreaks 2007/2008 and affected species
Year | Month | Cattle | Sheep | Goats | Wildlife | Total |
|---|---|---|---|---|---|---|
2007 | May | – | – | – | – | 0 |
2007 | June | 2 | – | – | – | 2 |
2007 | July | 10 | 8 | – | – | 18 |
2007 | August | 979 | 1,253 | 6 | 3 | 2,241 |
2007 | September | 5,142 | 4,792 | 60 | 21 | 10,015 |
2007 | October | 3,916 | 1,621 | 42 | 29 | 5,608 |
2007 | November | 1,755 | 96 | 6 | 14 | 1,871 |
2007 | December | 836 | 20 | 1 | 14 | 871 |
2008 | January | 854 | 11 | 2 | 5 | 872 |
2008 | February | 572 | 9 | 4 | 1 | 586 |
2008 | March | 428 | 5 | 1 | 3 | 437 |
2008 | April | 122 | 1 | 1 | 3 | 127 |
Total | 14,616 | 7,816 | 123 | 93 | 22,648 | |
Table 5.3
Exposed, diseased and dead animals and morbidity, mortality and case-fatality rate of BT infections for Germany in 2006 and 2007
Outbreak season | May 2006–April 2007 | May 2007–April 2008 | May 2008–April 2009 | ||||||
|---|---|---|---|---|---|---|---|---|---|
Species | Cattle | Sheep | Goats | Cattle | Sheep | Goats | Cattle | Sheep | Goats |
Premises | 758 | 319 | 13 | 14,756 | 7,910 | 257 | 2,956 | 278 | 11 |
Animals kept on affected farms | 67,080 | 9,825 | 56 | 1,501,994 | 505,934 | 3,736 | 425,959 | 62,915 | 141 |
Diseased animals | 1,445 | 370 | 0 | 30,175 | 18,821 | 173 | 5,358 | 429 | 4 |
Dead animals | 84 | 222 | 0 | 3,664 | 13,337 | 54 | 94 | 238 | 1 |
Morbidity (%)a | 2.15 | 3.77 | 0.00 | 2.01 | 3.72 | 4.63 | 1.26 | 0.68 | 2.84 |
Mortality (%)b | 0.13 | 2.26 | 0.00 | 0.24 | 2.64 | 1.45 | 0.02 | 0.38 | 0.71 |
Case fatality (%)c | 5.49 | 37.50 | 0.00 | 10.83 | 41.47 | 23.79 | 1.72 | 35.68 | 20.00 |
To take potential underreporting of BT cases into account, we also determined the number of animals for which the owners received financial aid from the German animal disease compensation funds (Tierseuchenkassen). In total, 10,240 cattle, 33,233 sheep and 102 goats were compensated for in 2007. This indicates an overall mortality of 0.08% in cattle and 1.36% in sheep. By focussing on the core region (North Rhine-Westphalia), where a prevalence of up to 100% can be assumed (by comparison to the situation in Belgium; Gethmann et al. unpublished), the mortality was 0.51% in cattle and 13.19% in sheep.
While several member states declared vector-free periods according to Commission Regulation (EC) No. 1266/2007 of 26 October 2007 during the cold season, i.e. defined as a period where the risk of virus transmission is deemed extremely low or negligible, thus allowing for a temporary lift of some trade restrictions, transmission was shown in Schleswig-Holstein in February 2008 (Hoffmann et al. 2008). This indicates that vector transmission on a low level may have played a role in the overwintering mechanism of BT in Germany and supported the view that declaration of a seasonally vector-free period was not appropriate for Germany.
In order to control BTV-8, to reduce the suffering of BT-infected animals and to mitigate the economic damage caused by the epizootic, it was decided to conduct a compulsory vaccination programme in Germany using inactivated vaccines which had not yet been licensed when the programme started (for details, see Chap. 5.6.2 Vaccination). The vaccination programme started in May and led to a massive decrease of new outbreaks in 2008 (Conraths et al. 2009) (Fig. 5.7, Table 5.4).
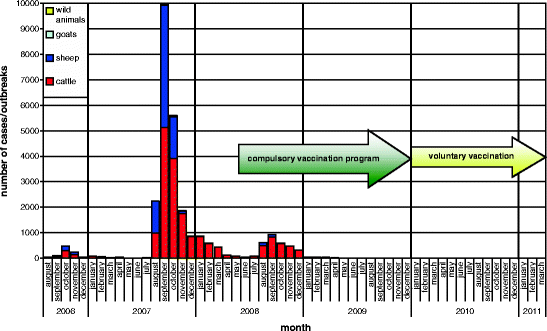

Fig. 5.7
Bluetongue disease, monthly incidence (source: TSN)
Table 5.4
Number of reported outbreaks 2008/2009
Year | Month | Cattle | Sheep | Goats | Wildlife | Total |
|---|---|---|---|---|---|---|
2008 | May | 70 | 3 | – | – | 73 |
2008 | June | 33 | 1 | – | – | 34 |
2008 | July | 73 | 13 | 1 | – | 87 |
2008 | August | 492 | 118 | – | 1 | 611 |
2008 | September | 819 | 111 | 2 | – | 932 |
2008 | October | 567 | 20 | 1 | 1 | 589 |
2008 | November | 461 | 3 | – | – | 464 |
2008 | December | 307 | 3 | – | 2 | 312 |
2009 | January | 39 | 2 | – | – | 41 |
2009 | February | 44 | – | – | 1 | 45 |
2009 | March | 34 | 1 | – | – | 35 |
2009
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|