Kelsey A. Hart
Blood Transfusion and Transfusion Reactions
Whole blood transfusion is an integral component of managing severe anemia in horses and foals. Although complete diagnostic evaluation and management of equine anemia can sometimes require specialized modalities that are available only in referral settings, much can be accomplished by an astute clinician in a field setting. This chapter reviews etiologies and diagnostic evaluation of anemia in horses and foals, discusses indications for blood transfusion (“transfusion triggers”), describes protocols for collection and administration of blood and blood products in horses, and examines mechanisms and management of transfusion reactions.
Pathogenesis and Evaluation of Anemia in Horses
Mechanisms of Anemia
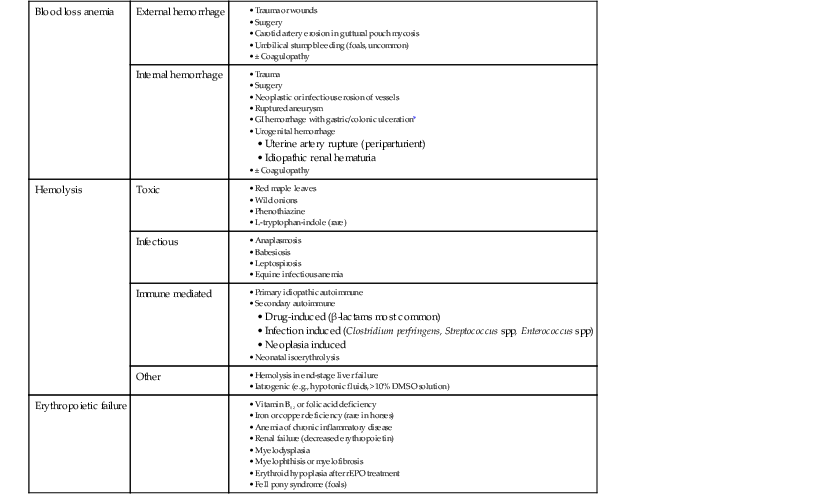
In any animal, three primary mechanisms can result in anemia: (1) blood loss (external or into a body cavity or organ), (2) hemolysis, or (3) impaired erythropoiesis (depression anemia). Common specific causes of anemia in horses and foals are detailed in Table 115-1. It is important to remember that in acute blood loss, an animal may develop signs related to hypovolemia and tissue hypoxia (e.g., tachycardia, tachypnea, or hyperlactatemia), even before anemia is actually detected on laboratory analysis. Typically, a decrease in plasma protein concentration is noted before a substantial decrease in the hematocrit is seen, from both movement of fluid from the extravascular space to maintain vascular volume and release of stored erythrocytes by stress-induced splenic contraction. A concurrent coagulopathy can contribute to external or internal hemorrhage and should be considered if blood-loss anemia is suspected. Animals with defects in primary hemostasis (e.g., thrombocytopenia or platelet dysfunction) tend to have signs of mucosal hemorrhage, such as epistaxis or hemorrhage in the gastrointestinal or urogenital tracts, whereas animals with defects in secondary hemostasis, such as clotting factor deficiencies, tend to hemorrhage from larger vessels and usually present with intracavitary hemorrhage (e.g., hemothorax).
Hemolytic anemia can develop from a variety of mechanisms. Primary idiopathic autoimmune anemia is not common in horses, but secondary immune-mediated hemolytic anemia (IMHA) related to drug treatment, infection, or neoplasia is an important differential in equine hemolytic anemia. Toxic mechanisms resulting in hemolytic anemia in horses most often cause Heinz body formation and erythrocyte lysis secondary to oxidative damage. It is also important to note that some infectious causes of hemolytic anemia, such as equine infectious anemia, actually can cause anemia through multiple mechanisms, including both intravascular and extravascular hemolysis as well as bone marrow suppression.
Depression anemia caused by impaired erythropoiesis at the level of the bone marrow is less common in horses than blood loss or hemolysis. The most common form of depression anemia in horses is anemia of chronic inflammation, which develops secondary to sequestration of iron, general bone marrow depression, and decreased erythrocyte life span, all mediated by inflammatory cytokines. This results in a mild to moderate, normocytic normochromic anemia that does not typically necessitate specific therapy. Anemia associated with deficiencies of iron, copper, vitamin B12, or folate can be seen in malnourished horses, animals with intestinal malabsorptive disease, or animals on long-term pyrimethamine or sulfonamide therapy (caused by folic acid inhibition). Other causes of bone marrow failure, such as myelodysplasia or myelophthisis, are uncommon but should not be overlooked.
Diagnostic Evaluation of the Anemic Patient
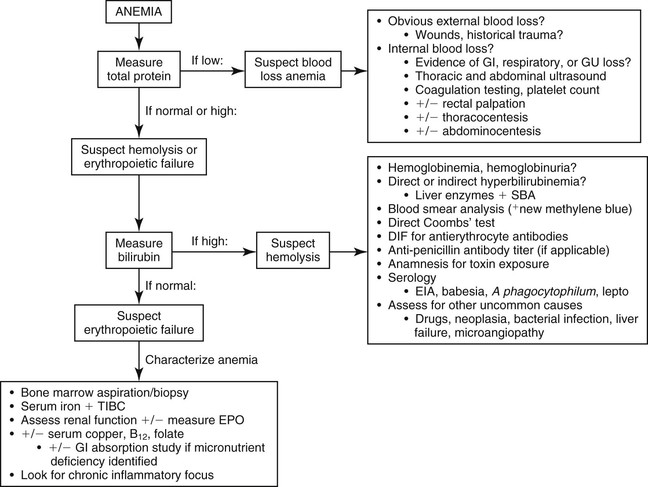
A general diagnostic plan for evaluation of anemia in horses that have undergone colic surgery is outlined in Figure 115-1. The primary step in this process involves evaluation for hemorrhage as a contributing cause of the anemia by assessment of hematocrit and total plasma protein (TP) concentration. Blood loss anemia is typically characterized by anemia in conjunction with low TP concentration, whereas anemia with an appropriate TP concentration is consistent with hemolysis or erythropoietic failure. Occasionally, however, hemolytic or depression anemia that develops with concurrent gastrointestinal tract or renal protein loss can initially manifest as low hematocrit and low TP, mimicking blood-loss anemia. This possibility should be considered if a source of external or internal blood loss cannot be identified.
The hallmark of hemolytic anemia is evidence of erythrocyte destruction intravascularly, extravascularly, or both. Intravascular hemolysis results in hemoglobinemia with or without hemoglobinuria, usually characterized by pink or red plasma or urine. Extravascular hemolysis typically results in an indirect (unconjugated) hyperbilirubinemia, although in foals with neonatal isoerythrolysis (NI), high values for both conjugated and unconjugated bilirubin are often seen. Clinical icterus is apparent if total bilirubin concentration is more than 1.5 to 2 mg/dL. Moderate to marked indirect hyperbilirubinemia also occurs with as little as 24 to 48 hours of fasting, complicating interpretation of serum bilirubin concentrations in anemic, anorexic horses. Liver disease must also be ruled out as a cause of hyperbilirubinemia. Finally, if hemolysis occurs at a slow rate or the hematocrit is already very low, hyperbilirubinemia or hemoglobinemia may not be seen.
Cytologic evaluation of erythrocyte morphology is important in suspected hemolytic anemia; spherocytosis is consistent with IMHA, whereas the presence of Heinz bodies or erythrocyte parasites supports toxic and infectious causes of hemolysis, respectively. Direct Coombs’ test and direct immunofluorescent antibody testing for antierythrocyte antibodies should be performed when IMHA is suspected. However, false-negative results can occur, especially as hemolysis progresses, when most antibody-coated cells are already lysed and removed from the circulation. A lack of antierythrocyte antibodies in hemolytic anemia can also indicate exposure to hemolytic toxins or to infectious causes.
Erythropoietic compromise should be considered in anemic horses when blood loss and hemolytic anemia have been ruled out and in those lacking a timely and appropriate regenerative response to other causes of postoperative anemia. In general, the hematocrit should begin to increase within 5 to 7 days of cessation of hemorrhage or hemolysis, and should return to reference intervals within about 30 to 45 days. Evaluation of a bone marrow aspirate or biopsy specimen is necessary in horses to determine whether an appropriate erythroid regenerative response is occurring because peripheral signs of regeneration (macrocytosis, polychromasia, and reticulocytosis) are not seen in horses. If an appropriate erythroid regenerative response is apparent on bone marrow evaluation, reevaluation for occult internal blood loss and hemolysis is warranted.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree