Chapter 23 Basic Water Quality Evaluation for Zoo Veterinarians
Of the various marine organisms, only marine mammals have specific government enforced regulations. The Animal and Plant Health Inspection Service (APHIS) has water quality standards for coliform count, pH, chemical additives, and salinity. Although these are the only taxa and parameters that are regulated, all other organisms need specific requirements. The first step in understanding these elaborate processes is to appreciate the complexities of water chemistries and some of the more advanced techniques to keep the parameters within acceptable biologic limits. Although it is easy to focus on one parameter that is out of range, it is imperative that the clinician examine each chemical in context with the bigger picture of chemistries. For example, temperature and pH greatly influence other values, such as ammonia levels. Determining only selected chemistry values may give the veterinarian a partial view and false impression of the situation, possibly resulting in improper action. Parameters for all aquatic animals other than mammals and birds include specific basic measurements, such as temperature, pH, dissolved oxygen (DO), ammonia, nitrite, and nitrate levels, salinity (conductivity), hardness, and alkalinity, but there are also more detailed analyses that may need to be carried out if these parameters are within normal limits (see Chapter 25). These may include oxidation-reduction potential (ORP), total organics, metals, and organic contaminants, which may involve more specialized equipment, such as mass spectrophotometers, total organic carbon analyzers, and ORP analyzers. These are not readily found in the zoo setting, so reference laboratories may need to be used.
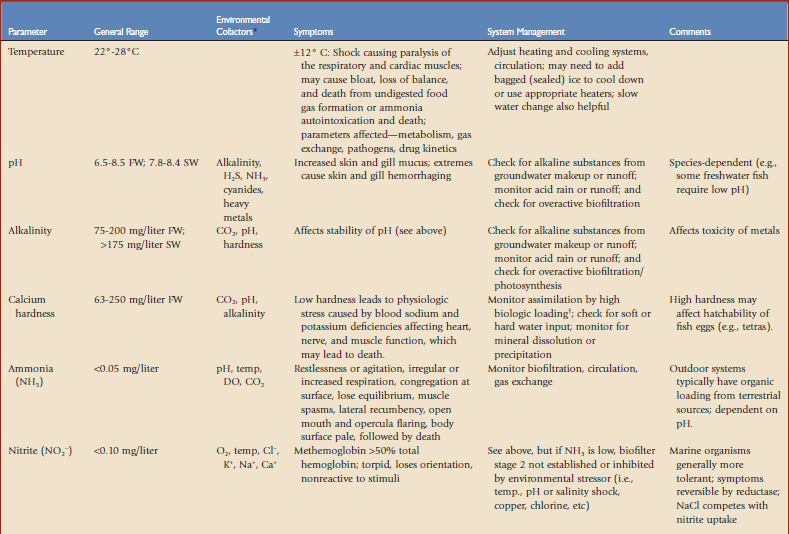
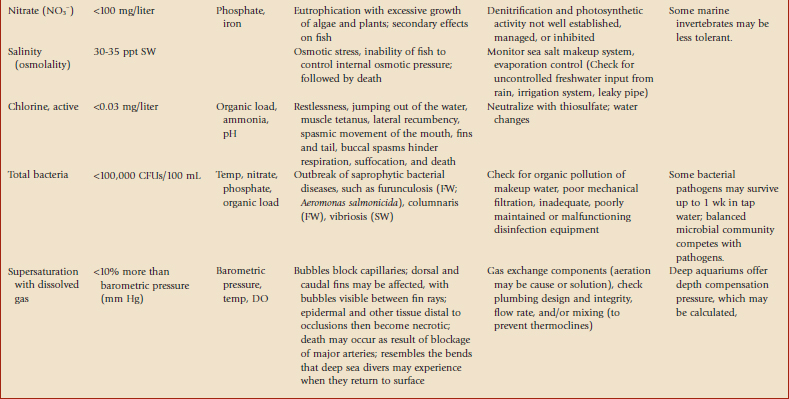
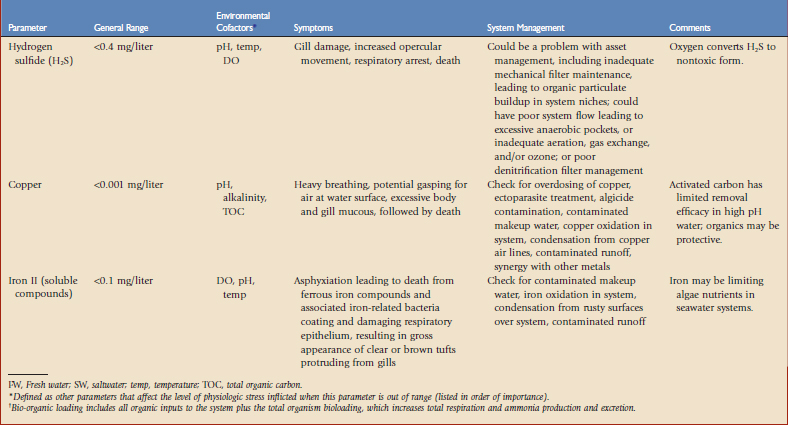
This chapter is intended to be a primer. Most discussions will focus on marine systems because of their complexity, but variations with freshwater will be highlighted. Many studies of aquatics have been presented in the literature but most do not mention water quality in significant detail. Tables 23-1 and 23-22 and Figure 23-1 provide basic information about recognizing water quality–related health issues and how to address these problems. For more information on general water quality,* we recommend studies by Spotte,16 Clesceri and colleagues,4 and Mohan and Aiken.13
TABLE 23-2 Water Quality Emergencies
| Emergency Kit Item | Event | Instructions |
|---|---|---|
| Chemical Treatment | ||
| Sodium thiosulfate solution (0.2 g/mL)* | Total Cl > 0.05 mg/L | One drop/gallon treats 0.95 mg/L |
| AmQuel solution (0.6 g/mL)* | NH3-N > 0.50 mg/L | 1 mL/gallon treats 1 mg/L |
| Zeolite ammonia remover | NH3-N > 0.50 mg/L in a freshwater system | 5.7 g (1 tsp)/gallon treats 1 mg/L |
| Sodium chloride | NO2-N > 1.00 mg/L in a freshwater system | 11 g (2 tsp)/gallon |
| 1 : 6 baking soda–soda ash buffer* | pH > 0.3 below acceptable level | Add 2 tsp/gallon, then as needed |
| pH-decreasing powder | pH > 0.3 above acceptable level | Follow directions on bottle |
| Granulated activated carbon (GAC)* | Toxic levels of heavy metals, Cl, ozone, medications, pesticides, organic contaminants, unknown toxins | Use 454 g (1 lb)/200 gallons (GAC) |
| Hydrogen peroxide (3%) | DO < 2 mg/liter | Use 5 mL/gallon |
| Equipment | ||
| Water weld | System leak in tank or pipe | Makes repairs under water in minutes |
| Portable canister filter | To use with GAC or zeolite | Filter water for toxic substances |
| AC/DC air pump(s) with air stones, air tubing, diffuser stones | Poor gas exchange caused by power outage | Aerate(s) system |
| Scuba tank with air line regulator | Same as above | Same as above |
| Appropriate transport containers, system nets, and holding tanks with adequate life support to accommodate all system animals | To rescue fish from major system failure or a water contamination event | Transport fish to the holding system |
| Reserve system water for a 100% water change | Major water contamination or loss | Replace or change out system water |
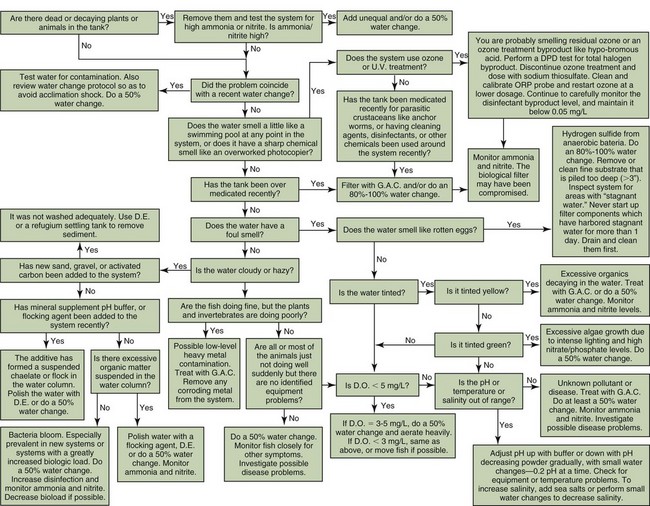
Figure 23-1 Algorithm for trouble-shooting water challenges. DE, Diatomaceous earth; GAC, granulated activated carbon.
Water Parameters and Testing
Sample Collection
Accurate water analysis begins with proper sampling protocol. Consideration of contamination issues and water collection technique standardization are critically important for accurate results. For more information on techniques, see the study of Clesceri and associates.4 When faced with an unknown water quality condition, always save water prior to changing the situation. Approximately 500 mL of water should be frozen for further chemical analysis if first-round diagnostics do not answer the question. Other more elaborate preservation methods are necessary for longer storage periods and are specific to the types of analyses to be conducted. Check the analyzer manuals for these preservation techniques.
Test Methods
Test methods that are commonly used in the zoo and aquarium industry include colorimetric, titrimetric, electrometric, nephelometric, and thermometric methods. A variety of test kits are available; some use test strips, some use wet reagents, and others use dry reagents. Although the test strips are handy for quick checks, they are not as reliable as kits that use dry and wet reagents or bench top analyzers. Before acquiring a kit, make sure that it contains reagents that have expiration dates. For detailed explanations of these tests, see report by Clesceri and coworkers.4
Water Quality Parameters and Analytic Testing for Marine Animals
Temperature
Water temperature also has a great influence on the initiation and course of a number of fish diseases. The immune system of most fish species studied has an optimum performance at water temperatures of approximately 15° C. At the other extreme, low temperatures slow metabolism, depress the immune response, and impair digestion. Poikilothermic organisms tend to be more severely affected in terms of physiologic stress by rapid temperature fluctuations rather than by large gradual changes within acceptable limits for the species. Temperature changes should be gradual, ideally no more than 2° C/hr.19 As a general rule, high temperatures also promote more rapid growth and reproduction of parasites and bacteria.
Dissolved Oxygen
DO may be measured with an electrode system wherein the dissolved oxygen reacts at the cathode, producing a measurable electrochemical effect. DO is more soluble at lower temperatures and salinities. Every aquarium system has a certain demand for oxygen that is created by the life that is present, known as biologic oxygen demand (BOD). The BOD is high when a large amount of organic matter is present that serves as food for bacteria and other microorganisms. The nitrifying bacteria that live in the biologic filter are aerobic and, as such, they are also adversely affected by DO lower than 2 mg/liter.7 It is important to note that oxygen may be the principal factor limiting biologic filter efficiency, and that its carrying capacity may be increased by almost 30% by using prefiltration techniques to remove gross and dissolved organics, thereby reducing the oxygen consumed in filtration.12 It is also important to make sure that the air supply is safe. Some operations have air intakes that may be in another building and pumped throughout an aquarium. Always examine the area for potential exhaust intake. Diesel exhaust or even a smoking area within the vicinity of the intake may be lethal to fish and would be difficult to detect with the usual testing methods in a zoo or aquarium. This would also apply to pesticides or painting that might be done in the area.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree