CHAPTER 4 Bacterial Skin Diseases
Cutaneous bacteriology and normal defense mechanisms
The skin forms a protective barrier without which life would be impossible. The defense has three components: physical, chemical, and microbial (see Chapter 1).4,7
The skin is an immune organ that plays an active role in the induction and maintenance of immune responses, which can be beneficial or detrimental (see Chapter 8). Specific components include epidermal Langerhans cells, dermal dendrocytes, keratinocytes, skin-seeking T-lymphocytes, mast cells, and the endothelium of postcapillary venules. Various cytokines, complement, and immunoglobulins are found in the emulsion layer and contribute to the skin’s immunologic function. Many individual components of this complicated system have antimicrobial effects, so the normal skin should be viewed as an organ that is resistant to infection.
Bacteria cultured from normal skin are called normal inhabitants and are classified as resident or transient, depending on their ability to multiply in that habitat (see Chapter 1). Residents successfully multiply on normal skin, thus forming a permanent population that can be reduced in number by degerming methods, but not eliminated. Transients are cutaneous contaminants acquired from the environment and can be removed by simple hygienic measures.
Studies on the microbial flora of normal equine skin have been strictly qualitative (see Chapter 1). It is clear that skin and hair coats are exceedingly effective environmental samplers, providing a temporary haven and way station for all sorts of organisms. Thus, only repetitive quantitative studies will allow reliable distinction between equine cutaneous residents and transients. Coagulase-negative Staphylococcus sciuri and S. xylosus are frequently isolated from the skin and nostrils of normal horses and may be resident bacteria.7,42,43,56
There has been speculation about the means by which only a small number of a vast array of bacteria in the environment are able to colonize or infect the skin. The potent cleaning forces of dilution, washout, drying, and desquamation of surface cells prevent many organisms from colonizing the skin. It is now recognized that bacterial adhesion is a prerequisite to colonization and infection.4,7 Bacterial adhesion is a complex process influenced by both the host and the organism. Bacteria possess surface adhesion molecules, which influence their ability to bind to keratinocytes. For staphylococci, teichoic acid and protein A appear to be most important surface adhesion molecules. These molecules bind to host surface receptors (e.g., fibronectin and vitronectin) to prevent the bacteria from being brushed from the skin. Adhesion is increased with increasing time, temperature, and concentration of bacteria, and in certain diseases. In hyperproliferative disorders, more bacteria adhere to the skin because more binding sites are available. Organisms from the transient group are pathogenic in rare cases. Gram-negative organisms tend to flourish in moist, warm areas and to predominate when medications depress the gram-positive flora.
Skin infections
The normal skin of healthy individuals is highly resistant to invasion by the wide variety of bacteria to which it is constantly exposed. Pathogenic organisms such as coagulase-positive staphylococci may produce characteristic lesions of folliculitis, furunculosis, and cellulitis in the absence of any obvious impairment of host defenses. However, localized disruption of normal host defenses as produced by maceration (water, friction from skin folds, topical treatments, increased humidity and/or friction associated with tack/blankets/riders), physical trauma (abrasions, cuts, punctures, biting insects and arthropods, scratching, and rubbing), or the introduction of a foreign body (plant awns) may facilitate development of overt infection. Treatment with immunosuppressive agents and immunosuppressive diseases can predispose patients to infections. Widespread bacterial skin infections in a herd of horses were associated with malnutrition and unhygienic environmental conditions.40
Because the skin of horses is a veritable cesspool of bacteria, cultures must be carefully taken and interpreted (see Chapter 1). Intact pustules, nodules, and abscesses are preferred lesions for culture and may be aspirated with a needle and syringe or punctured and swabbed with a culturette, after the overlying epithelium has been gently swabbed with alcohol and allowed to air-dry. Cultures of open sores (erosions, ulcers, and sinuses) and exudative surfaces often generate confusing, if not misleading, bacteriologic data.
Treatment of skin infections
Satisfactory resolution of a skin infection necessitates that the cause of the infection be identified and corrected and that the infection receive proper treatment.4-8,17 If the cause of the infection persists, either the response to treatment is poor or the infection recurs shortly after treatment is discontinued. If the cause is resolved but inappropriate treatment for the infection is given, the infection persists and worsens.
Skin infections can be treated topically, systemically, surgically, or by some combination of these. Some equine infections are too widespread or too deep to be resolved with topical treatment alone, but judicious topical therapy can make the patient more comfortable and hasten its response to antibiotics. Topical treatment can take considerable time and effort on the owner’s part and can irritate the skin if the products are too harsh. Surgery alone can be useful with focal lesions or can be performed as an adjunct to other treatments.1 Management must be individualized.
Topical Treatment
Topical treatments are used to reduce or eliminate the bacterial population in and around an area of infection and to remove tissue debris (see Chapter 3).2,6,7,10 Debris removal is of paramount importance because it allows direct contact of the active ingredient with the organism and promotes drainage. Agents commonly used include chlorhexidine, povidone-iodine, benzoyl peroxide, and various antibiotics, especially fusidic acid, mupirocin, and bacitracin.
Infections restricted to the skin surface or intact hair follicles may be effectively treated with topical agents alone. When the number of lesions is small and they are confined to a limited area, antiseptics or antibiotics in a cream, ointment, or gel formulation may be sufficient to resolve the infection (see Chapter 3). Benzoyl peroxide gels or antibiotic formulations receive widest use. The benzoyl peroxide gels marketed to veterinarians contain 5% active ingredient, which can be irritating, especially with repeated application. In most instances, antibiotic preparations are nonirritating. In most cases, transdermal absorption of the agent is limited, but frequent application over wide areas should be avoided.
Many potent antibacterial agents are available in topical form (see Chapter 3). The most commonly used are mupirocin, fusidic acid, and silver sulfadiazine (Silvadene).* Neomycin, gentamicin, bacitracin, and polymyxin B can also be effective.7 Important considerations for some of these agents are as follows: (1) mupirocin and fusidic acid are more effective than other topical agents for treatment staphylococcal pyodermas; (2) mupirocin has poor activity against gram-negative infections; (3) neomycin has more potential for allergic sensitization than do most topicals, and susceptibility is variable for gram-negative organisms; and (4) polymyxin B and bacitracin in combination may be effective for gram-negative and gram-positive organisms; however, they are rapidly inactivated by purulent exudates and do not penetrate well. Mupirocin 2% ointment is particularly useful because of its ability to penetrate the skin and its very low incidence of adverse reactions.6,7 It is inactivated by exudates and debris, so the surface of lesions must be cleaned prior to application. Silver sulfadiazine 0.1% cream is broad-spectrum, penetrating, and usually well-tolerated.
A 0.4% stannous fluoride (MedEquine) (broad-spectrum antibacterial agent) gel was applied every 24 h for 4 weeks to bacterial folliculitis lesions in horses.20 The study was placebo-controlled and double-blinded. The stannous fluoride was significantly more effective than placebo, and no adverse effects were reported.
Widespread superficial infections are best treated with antibacterial shampoos (see Chapter 3).* The manipulation of the skin during its application and the vehicle of the shampoo removes tissue debris, which allows better contact between the antiseptic and the bacteria. Product selection depends on the preferences of the owner and the clinician and the condition of the animal’s skin. Animals with underlying hypersensitivity disorders or “sensitive” skin should be bathed with nonirritating or minimally irritating agents such as chlorhexidine (see Chapter 3). Benzoyl peroxide products should be reserved for greasy horses or horses with deep crusted infections (see Chapter 3). In this latter group, shampoo selection should be reevaluated in 10-14 days because the skin will be much different then.
Iodophors are popular topical antimicrobial agents in equine practice.2,4,7,10 Povidone-iodine is available as a 5% solution (Betadine, Purdue Frederick; Poviderm, Vetus; Povidone-Iodine, Equicare), a 5% shampoo (Poviderm, Vetus; Povidone, Butler), and a 10% ointment (Povidone Iodine, First Priority). Polyhydroxydine complex iodine is available as a 1% solution and a 1% spray (Xenodine, V.P.L.). Although these products are excellent antimicrobial agents, their propensity for causing dry, scaly skin and hair coat and irritation, and possible staining of skin and hair coat makes them less desirable than chlorhexidine or benzoyl peroxide.
Systemic Antibiotics
Systemic antibiotic agents are used for bacterial skin diseases that are not treatable with topical therapy.2,4-10,17,21 Appropriate systemic antibiotic use in the equine presents many challenges. Specific considerations include: poor oral absorption of many drugs; large total dosage and, therefore, high cost; risk of side effects, the most common of which is enterocolitis; extralabel use of drugs due to lack of licensed equine products; and differences in drug disposition in foals versus adults. In addition, there is the concern over development of antimicrobial resistance, which has important implications for both veterinary and human medicine.
Knowledge and understanding of basic pharmacokinetics and pharmacodynamics are essential. In addition, controversies exist regarding the current use of antibiotics, including appropriate selection and use in certain clinical situations. The reader is encouraged to consult available detailed information on these subjects.4-6,8,17,18
Penicillins
Penicillins are a good choice when bacteria are susceptible (Table 4-1).2,4-10,17 They are bactericidal, narrow-spectrum, time-dependent, and have a low incidence of severe side effects. They are a common cause of drug-induced urticaria (see Chapter 8), occasional colitis, and rare anaphylaxis and hemolytic anemia. Most streptococci, Corynebacterium pseudotuberculosis, Pasteurella spp., many anaerobes, many actinomycetes (Dermatophilus, Actinomyces), and Actinobacillus spp. are susceptible. Penicillins penetrate poorly into abscesses and necrotic tissue. Most coagulase-positive staphylococci are resistant. Potassium penicillin G seems to be interchangeable with procaine penicillin G. Procaine is eliminated slowly and commonly causes violative residues in race horses and performance horses.4
Trimethoprim-Potentiated Sulfonamides
Trimethoprim-potentiated sulfonamides are effective and popular antibiotics in equine dermatology (see Table 4-1).2,4-10,17 They are broad-spectrum, bactericidal, concentration-dependent, and generally well-tolerated, but they are a common cause of drug-induced urticaria, erythema multiforme, exfoliative dermatitis, and allergylike pruritus (see Chapters 8 and 9). They are inactivated in abscesses and necrotic tissues. Anemia and/or leukopenia may occasionally be seen in horses treated with long-term trimethoprim-potentiated sulfonamides. Monthly hemograms are recommended during prolonged therapy. Trimethoprim-sulfadiazine (30 mg/kg every 24 h) given PO to healthy horses produced no significant effects on serum concentrations of total and free thyroxine (T4), total and free triiodothyronine (T3), and thyrotropin (TSH).24 Most coagulase-positive staphylococci, streptococci, Dermatophilus congolensis, and many Actinobacillus spp., Rhodococcus equi, and C. pseudotuberculosis are susceptible.
Macrolides
Macrolides are narrow-spectrum, bacteriostatic, concentration-dependent, and have a long postantibiotic effect (see Table 4-1).* They concentrate in phagocytic cells and can be particularly effective for intracellular bacteria and in pyogranulomatous lesions. Most coagulase-positive staphylococci, streptococci, Pasteurella spp., Clostridium spp., Actinobacillus spp., and R. equi are susceptible. Macrolides are not used in adults due to the occurrence of severe and sometimes fatal colitis. In foals, macrolides may produce distress syndromes. Hence, caution is in order when treating foals in hot weather.
Macrolides (erythromycin estolate, clarithromycin) and the macrolide derivative azalides (azithromycin), combined with rifampin, are the treatment of choice for foals with R. equi infections. Clarithromycin is now available as a generic, is cost-effective, is better tolerated than erythromycin, and when combined with rifampin is clinically superior to either erythromycin- or azithromycin-rifampin combinations.28
Fluoroquinolones
Fluoroquinolones are bactericidal, broad-spectrum, concentration-dependent antibiotics with a long postantibiotic effect.2,4-10,17 They are concentrated intracellularly in phagocytes. Although the pharmacokinetics and pharmacodynamics of a number of fluoroquinolones have been reported in horses (e.g., marbofloxacin, orbifloxacin),4,13,15,23 only enrofloxacin is widely used in equine dermatology (see Table 4-1).2,4-10,17,26 Ciprofloxacin is contraindicated in horses due to poor PO absorption and possible severe colitis.
Enrofloxacin and its metabolite, ciprofloxacin, can be detected in equine mane and tail hairs at least 9 months after a 2-week course of treatment.37 Interestingly, concentrations are much higher in black versus white hairs, and enrofloxacin is extensively bound to melanin in vitro.37 Serum concentrations of enrofloxacin may be higher when certain feeds that contain high concentrations of divalent cations (e.g., alfalfa) are withheld pre- and 1-2 h postantibiotic administration.* Coagulase-positive staphylococci, many streptococci, C. pseudotuberculosis, and Actinobacillus spp. are susceptible. Most anaerobes are resistant.4,8,16 Enrofloxacin/β-lactam combinations are synergistic. Enrofloxacin is generally well-tolerated, but is contraindicated in horses less than 2-years old due to chondrotoxicity. Enrofloxacin is administered PO as the bovine injectable (horses object to the taste; rinse mouth with water after dosing) or the canine tablets (crush and mix with feed). When the bovine injectable was compounded in a gel, oral ulcers were seen in about 10% of the horses treated.11,16,17
Rifampin
Rifampin is a bactericidal, narrow-spectrum antibiotic that enters phagocytic cells and is effective for intracellular bacteria and in pyogranulomatous, abscessed, fibrosed lesions.2,4-10,17,27 Coagulase-positive staphylococci, streptococci, R. equi, C. pseudotuberculosis, and most anaerobes are susceptible. Because resistance to rifampin monotherapy can occur frequently and rapidly, it is usually given in conjunction with other antimicrobial agents (e.g., macrolides, β-lactams, trimethoprim-potentiated sulfonamides). Urine, tears, saliva, sweat, and clothing may be stained red/orange.
Gentamicin
Gentamicin is occasionally used in equine dermatology (see Table 4-1).2,4-10,17,27 It is bactericidal, broad-spectrum, concentration-dependent, and has an intermediate postantibiotic effect. Gentamicin has poor activity in abscesses and necrotic tissue. Coagulase-positive staphylococci, Pseudomonas spp., Actinobacillus spp., and R. equi are usually susceptible. Anaerobes are not susceptible. The risk for nephrotoxicity increases with prolonged therapy (>7-10 days) and with preexisting renal disease. This risk can be decreased by feeding a high protein, high calcium diet (such as alfalfa).4
Cephalosporins
Cephalosporins are excellent broad-spectrum, bactericidal, time-dependent antibiotics with an intermediate postantibiotic effect that are very useful against coagulase-positive staphylococci, streptococci, Pasteurella spp., Salmonella spp., and anaerobes (not Bacteroides spp. and Enterococcus spp.) (see Table 4-1).* They are generally well-tolerated. β-lactams are synergistic with aminoglycosides and fluoroquinolones. Ceftiofur is effective IM, intravenously (IV), and subcutaneously (SQ) (and registered for the horse), and cephalexin is effective PO.
Chloramphenicol
Chloramphenicol is a bacteriostatic, broad-spectrum, concentration-dependent antibiotic with a long postantibiotic effect (see Table 4-1).2,4-6,17 It has good activity against staphylococci. It is generally well-tolerated. Chloramphenicol is not to be used in food animals, due to a severe, idiosyncratic bone marrow dyscrasia that occurs in humans.
Tetracyclines
Tetracyclines are bacteriostatic, broad-spectrum, concentration-dependent antibiotics with a long postantibiotic effect (see Table 4-1).2,4-6,17 They have variable activity against staphylococci and streptococci. The use of oxytetracycline is controversial, but it is used successfully and increasingly in equine practice (IM use only).2,4,17 Doxycycline is well-absorbed PO, concentrated intracellularly in phagocytic cells, and generally well-tolerated. It is generic and inexpensive. Feed may reduce PO bioavailability.6
No discussion of antibiotics would be complete without mentioning some of the antiinflammatory and immunomodulatory properties inherent to some of these agents: macrolides (inhibit leukocyte chemotaxis, interleukin (IL)-1, and lymphocyte blastogenesis), trimethoprim (inhibits leukocyte chemotaxis), and fluoroquinolones (inhibit IL-1, leukotriene, and tumor necrosis factor (TNF)-α synthesis; inhibit granulomatous inflammation).7 These effects can be beneficial but may also be misleading.
Immunomodulatory Agents (Biologic Response Modifiers)
Horses with recurrent, unexplained skin infections, wherein clinical and laboratory findings are normal when the infections are eliminated with antibiotic therapy, pose a difficult therapeutic problem. Weekly or biweekly antibacterial shampoos and/or rinses may help reduce the frequency of relapses.7 If topical treatments are ineffective, and recurrences are not too frequent (e.g., twice or thrice a year), appropriate systemic antibiotic therapy may be satisfactory. More frequent episodes of infection, or inability to use antibiotics, may cause the veterinarian to consider immunomodulatory therapy.
There is growing interest in developing preparations that augment immune defenses to prevent and treat infectious diseases. Immunostimulant preparations produce nonantigen-specific enhancement of cellular and humoral defense mechanisms, presumably through amplification of phagocytosis and intracellular killing by neutrophils and macrophages, antigen presentation, cytotoxic activity of T-lymphocytes and killer cells, cytokine release, and antibody production.4-6,19,25 The effectiveness of such products depends on the horse’s own ability to respond with the production of IL-1, IL-6, IL-15, IL-18, TNF-α, and interferon (IFN). Common systemic reactions to such products include mild fever and depression. In equine medicine, immunostimulant preparations are used predominantly for treatment of chronic respiratory disease and sarcoids (Box 4-1). These preparations are also being used in horses with various dermatoses, infectious and noninfectious, on a completely empirical and anecdotal basis. The authors have used none of these products for treating bacterial dermatoses in horses.
Box 4-1 Immunomodulatory Agents
Bacterial, Viral, and Plant Products
Levamisole (Levasole): not registered in horses; 2 mg/kg every 48 h PO.
Autogenous bacterins have been reported to be helpful in recurrent bacterial dermatoses in horses.2,7 All such reports are anecdotal.
Staphylococcal infections
Prior to 1980, all coagulase-positive staphylococci isolated from horses were identified as Staphylococcus aureus. Currently, three coagulase-positive staphylococci are known to be associated with equine skin infections: S. aureus, S. hyicus subsp. hyicus, and S. delphini.* Prior to 2005, S. intermedius was frequently isolated from equine infections.7 Advances in molecular biology have demonstrated that the S. intermedius complex actually includes three organisms: S. intermedius (wild pigeons), S. pseudintermedius (dogs, cats, humans), and S. delphini (horses and domestic pigeons).36,47,48 It is highly likely that all equine isolates of “S. intermedius” reported prior to 2007 were actually S. delphini.
It is not presently clear whether or not the different staphylococci vary in frequency of isolation from equine pyogenic dermatoses, or are always associated with particular clinical syndromes and epidemiologic scenarios. For instance, some reports state that S. aureus is the major Staphylococcus isolated from equine dermatoses, and that “S. intermedius” (S. delphini) is rarely isolated, while other reports indicate that S. aureus and “S. intermedius” (S. delphini) or S. aureus and S. hyicus are isolated with about equal frequency.7
The coagulase-positive staphylococci produce various combinations of enterotoxins (A, B, C, D), protein A, hemolysins, leukocidins, and dermonecrotoxins that may be involved in the pathogenesis of infections.4,7,8 Protein A and enterotoxin C, in particular, are capable of acting as superantigens (see Chapter 8) and triggering local cutaneous and immunologic responses.36 S. aureus strains isolated from three horses with cellulitis in Japan did not produce enterotoxins A, B, and C.7 A S. aureus strain isolated from a horse with cellulitis in Japan produced an exfoliative toxin (“exfoliatin”), which produced generalized exfoliation when injected into 3-day-old mice and 1-day-old chicks.7 The toxin was, thus, similar to that associated with so-called staphylococcal scalded skin syndrome in humans. However, the horse from which the exfoliation-producing S. aureus was isolated had no clinical exfoliation. The following is a discussion of some of the clinical syndromes associated with staphylococcal infection in equine dermatology.
Folliculitis and Furunculosis
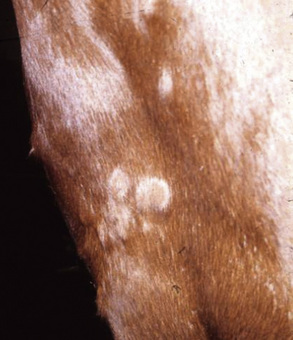
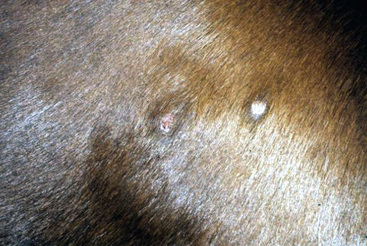
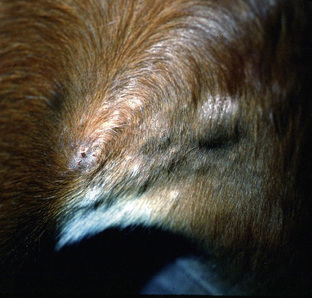
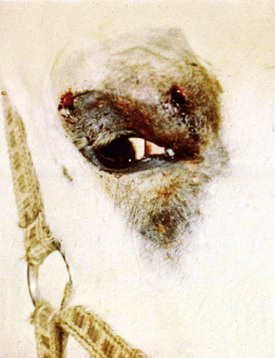
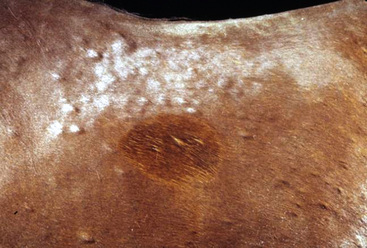
The primary skin lesion of folliculitis is a follicular papule. Pustules may arise from these papules. However, pustules are rarely seen in bacterial folliculitis. Frequently, one first notices erect hairs over a 2- to 3-mm papule that is more easily felt than seen (Figs. 4-1 and 4-2). Clusters of hairs stick up against the lie of the coat and may be glued together by small crusts. These lesions can regress spontaneously but often progressively enlarge. Some lesions enlarge to 6-10 mm in diameter, develop a central ulcer that discharges a purulent or serosanguineous material, and then become encrusted. The chronic or healing phase is characterized by progressive flattening of the lesion and a static or gradually expanding circular area of alopecia and scaling (Fig. 4-3). Hairs at the periphery of these lesions are often easily epilated. Epidermal collarettes are uncommonly seen (Fig. 4-4). It is extremely important to remember that in the chronic or healing stage, all folliculitides, regardless of cause, are often characterized by circular areas of alopecia and scaling (so-called classic ringworm lesion).
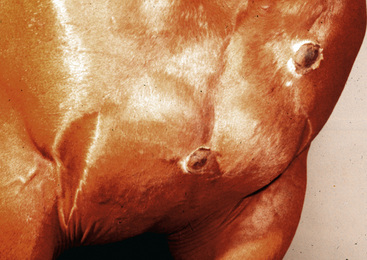
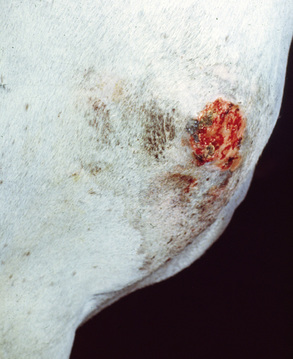
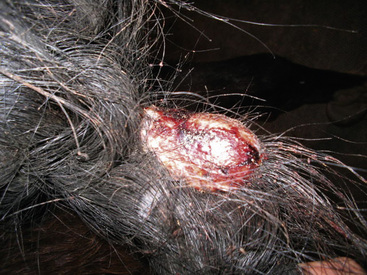
Some lesions progress to furunculosis. This stage is distinguished by varying combinations of nodules, draining tracts, ulcers, and crusts (Figs. 4-5–4-7). Large lesions are often associated with severe inflammatory edema and may assume an edematous plaque or urticarial appearance. Cellulitis may occur. Lymphatic engorgement may lead to the development of “runners” on the body wall radiating away from lesions. Scarring, leukoderma, and leukotrichia may follow.
Staphylococcal folliculitis and furunculosis (acne, heat rash, summer rash, summer scab, sweating eczema of the saddle region, sweat spots, saddle scab, saddle boils) are common.* Bacterial folliculitis (cocci phagocytosed by neutrophils seen on cytology, cultures usually not done) accounts for 11.8% of the equine dermatology cases seen at the CUHA. No age, breed, or sex predilections are evident. Most cases begin in spring and early summer. This period coincides with shedding, clipping, heavy riding and work schedules, higher environmental temperature and humidity, and increased insect population densities. Poorly groomed horses may be at risk. Lesions can, of course, occur at any time of year, and on any part of the body, reflecting the many predisposing causes (hypersensitivities, ectoparasites, trauma, filth, etc.).
Skin lesions initially affect the saddle and tack areas in about 90% of cases (Figs. 4-8–4-10). Hence, the sides of the neck, saddle region, rump, and shoulders are commonly affected. The superficial lesions of folliculitis are usually asymptomatic, while the deep lesions of furunculosis are often painful. Neither condition is commonly pruritic. If triggering factors are not recognized and eliminated, recurrences are common.
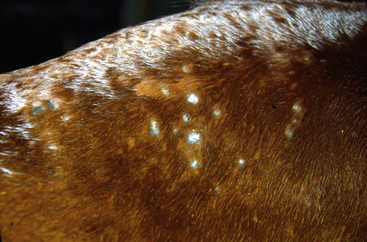
Figure 4-9 Staphylococcal folliculitis in saddle area. Annular areas of alopecia, scaling, and crusting.
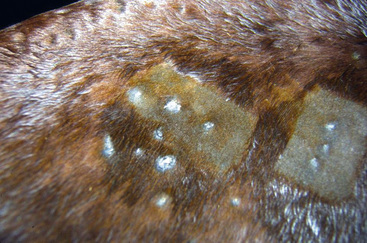
Figure 4-10 Same horse as in Fig. 4-9. Areas have been clipped to expose annular areas of alopecia and thick white scale-crust.
Although all coagulase-positive staphylococci have been isolated from folliculitis-furunculosis lesions, there is conflicting information as to which species is most common. Some clinicians indicate that S. aureus is most common, while others find S. hyicus subsp. hyicus most common.7
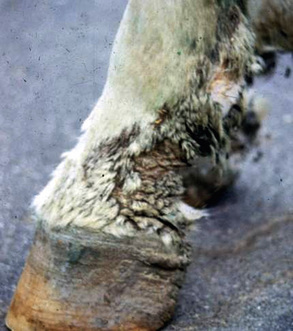
Pastern Folliculitis and Furunculosis
Bacterial infections may uncommonly be restricted to the caudal aspect of the pastern and fetlock regions, with involvement of one or more limbs (Fig. 4-11).2,7,9,10 This disorder must be considered in the differential diagnosis of “grease heel” or “scratches” (see Pastern Dermatitis, Chapter 15). Bacterial infections may, of course, be superimposed on other dermatoses of the pastern (vasculitis, dermatophytosis, dermatophilosis, chorioptic mange, contact dermatitis, etc.). Although all coagulase-positive staphylococci have been isolated from pastern folliculitis—sometimes more than one species simultaneously—there is conflicting information concerning the most commonly isolated species. A Japanese study found that S. aureus was most commonly isolated, while a Belgian study found S. hyicus subsp. hyicus most commonly.7 Infections were produced in normal horses by scarifying skin and applying S. hyicus inocula from clinical cases.
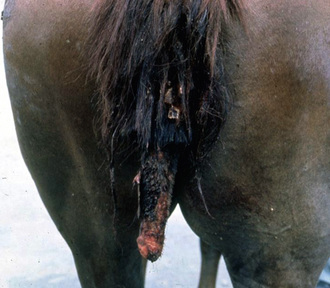
Tail Pyoderma
So-called tail pyoderma—actually folliculitis and furunculosis of the tail (Figs. 4-12 and 4-13)—usually follows the cutaneous trauma produced by tail rubbing provoked by insect-bite hypersensitivity, atopic dermatitis, food allergy, chorioptic mange, psoroptic mange, pediculosis, oxyuriasis, and behavioral abnormalities (vice).7
Cellulitis
Cellulitis (phlegmon) is a severe, deep diffuse suppurative infection wherein the process spreads through the dermis and subcutis along the tissue planes. The infection may extend to the skin surface, producing draining tracts. There may be extensive edema and swelling. The overlying skin may be friable, darkly discolored, and devitalized. Affected tissues may slough, leaving large ulcers. Many cases of leg cellulitis do not have a history of recent trauma (penetrating wound, surgical incision, injections), and are termed “primary” (idiopathic).9,29,38 Coagulase-positive staphylococci are isolated in greater than 80% of the cases.7,29,38 Streptococcus spp., E. coli, Enterobacter spp., and others are isolated much less commonly.
There are no apparent age or sex predilections, but Thoroughbreds and race horses account for the majority of reported cases.7,9,29,38 Animals typically develop acute swelling and lameness of one leg, and hind legs are affected more commonly. Most horses are febrile, and leukocytosis, neutrophilia, and hyperfibrinogenemia are common. Many horses develop laminitis in the contralateral leg.
Diagnosis is based on history, physical examination, and culture. Ultrasonographic imaging supports the clinical diagnosis and may provide useful information on the presence of fluid pockets than could be aspirated or surgically drained.29,38 Treatment needs to be instituted early and aggressively. Initial antimicrobial treatment often includes an IV β-lactam and aminoglycoside.29,38
Up to 25% of the horses with leg cellulitis are euthanized (necrosis and sloughing of tissue; severe laminitis).7,29,38 Where follow-up information was available, 69% and 77% of discharged horses were being used for their intended or original use or were sound, respectively.29 Recurrence of cellulitis occurred in 23% of discharged horses.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree