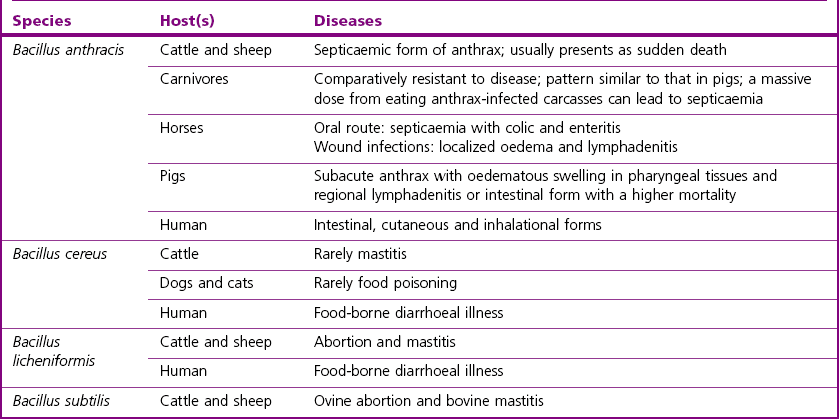
Chapter 14 Bacillus anthracis spores are geographically ubiquitous in soils. This bacillus most commonly infects ungulate herbivores. It has been reported that in nature the spores are associated with fairly heavy particles and are not likely to become airborne at carcass sites, despite rain, wind or some soil agitation (Turnbull et al. 1998). In nature, the vegetative form of B. anthracis seems to exist only in a host and is not found in the environment. Bacillus anthracis is considered the major animal pathogen in the Bacillus genus (Table 14.1) causing anthrax in herbivores and other mammals, including humans. In general, three main clinical forms have been described to date in the literature: cutaneous, gastrointestinal and respiratory (Hanna 1998). However, this classification is less useful in veterinary medicine, where anthrax is viewed as a singular disease that rapidly progresses to death without any specific clinical signs. It is often characterized by a massive septicaemia where hypotension, shock and sudden death are mainly attributed to the lethal toxin (LeTX). In contrast, in human cases of anthrax the symptoms precede death over many days. Animal anthrax is therefore difficult to treat because of the rapid onset of the condition. A mortality rate of approximatively 100% is reported for animal systemic infections. Susceptibility to the disease is variable among different animal species. Cattle, sheep and goats are most susceptible to infection, horses and humans occupy an intermediate position, while pigs, birds and carnivores are comparatively resistant, but can succumb if the infective dose is high. Disease has also occurred in bison, white-tailed deer, ostriches, mink and moose. Rats and some strains of mice are considered resistant. In cattle, sheep, and goats anthrax is considered a peracute disease characterized by septicaemia with high fever and sudden death (within one or two days). In some cases, the disease may last for about a week. Postmortem findings include exudation of tarry blood from body orifices, failure of the blood to clot, incomplete rigor mortis and splenomegaly in cattle. Indeed, a typical characteristic of anthrax is the failure of blood to clot following death. In the less susceptible species inflammatory subcutaneous oedema of face, throat and neck is a common finding and colic can occur in horses and gastroenteritis in carnivores. Sporadic disease and outbreaks have been observed in pigs where the condition was characterized by swelling of the throat and/or digestive disturbances with a low mortality rate (Edginton 1990, Williams et al. 1992). Research has suggested that meat from healthy pigs killed 21 days after the last case following an outbreak of anthrax should not pose a public health risk (Redmond et al. 1997). Anthrax occurs when endospores of B. anthracis gain entry to a host through ingestion, from soil when grazing or in contaminated food, through abrasions of the skin or following inhalation. Inhalation occurs to a lesser extent in animals than in humans. Transmission by biting insects may be important especially during an outbreak. Following entry, the endospores are rapidly phagocytosed by macrophages and then germinate inside the macrophages. Vegetative bacteria are released into the blood in which they rapidly multiply to high numbers. Virulence of most B. anthracis strains is associated with two megaplasmids (Table 14.2). Strains lacking either plasmid are avirulent or significantly attenuated. Plasmid pXO1 carries the genes for the anthrax tripartite protein toxin complex (Okinaka et al., 1999), while plasmid pXO2 carries the biosynthetic genes for the antiphagocytic poly-D-glutamic acid capsule. The anthrax tripartite toxin comprises three components: a protective antigen, a lethal factor and an oedema factor. These proteins act in binary combinations to produce the two anthrax toxins (Leppla 1995): oedema toxin (protective antigen and oedema factor) and lethal toxin (protective antigen and lethal factor). Capsule and toxin virulence factors seem to be regulated by host-specific signals such as CO2 concentration. Table 14.2 Main virulence factors of Bacillus anthracis Humans can incidentally acquire the disease by contact with endospores from infected animals or their contaminated products or from a bioterrorism source. About 95% of human anthrax cases are the cutaneous form, 5% respiratory, while the gastrointestinal form is very rare. There are no known cases of human-to-human transmission. Only a few endospores are required to cause cutaneous anthrax, while the infectious doses in gastrointestinal and respiratory forms are usually very high (50% lethal dose > 10,000 spores). Cutaneous anthrax cases are readily treated and become life-threatening only on exceptional occasions. The skin lesion starts with a pruritic papule which evolves to a painless black eschar (Dixon et al. 1999). The respiratory and gastrointestinal (GI) forms are both highly fatal forms of the disease if not treated. The gastrointestinal form usually occurs two to five days after the ingestion of spores from contaminated meat or food products. The respiratory form occurs after the inhalation of endospores usually by workers handling contaminated animal products or hides (Woolsorter’s disease). Endospores can remain dormant for more than 60 days in the lungs (Barakat et al. 2002). 1. asymetric septation of the vegetative bacilli (mother cell) 2. maturation of the forespore compartment 3. death of the mother cell surrounding the forespores Bacillus anthracis endospores are basically made of four structures: the core, the cortex, the spore coat, and the exosporidium. They contain protein, DNA, calcium, dipicolinic acid (DPA), a paracrystaline lipid bilayer, a thick cross-linked peptidoglycan and a collagen-like surface protein named BclA (Bacillus collagen-like protein of anthracis). Endospores are inert with no measurable metabolism and will not divide like vegetative cells. They have no ATP, no synthesis and very little or no water. The germination process occurs within the host and is triggered by various nutrient germinants which contact receptors on the spore itself. This causes the release of chelated Ca2+-dipicolinic acid from the spore core and hydration as water floods the core. Expulsion of DPA activates cortex lytic enzymes that degrade the spore cortex allowing germination to progress. The majority of the other Bacillus species have little or no pathogenic potential but can occur commonly as contaminants on laboratory media. Table 14.1 summarizes the main hosts and diseases of the pathogenic bacilli. Bacillus cereus is best known as a cause of food-borne illness in humans and occasionally affects animals, mainly dogs and cats (Chastain & Harris 1974). Bacillus cereus can produce two types of food-poisoning: the diarrhoeal type and the emetic type. Bacillus licheniformis is an important cause of bovine abortion and occasionally causes bovine mastitis. Bacillus subtilis has also been implicated in cases of food-poisoning and in cases of bovine mastitis and ovine abortion (Logan 1988). Bacillus coagulans has been isolated from bovine abortion cases whereas B. pumilus has been isolated from cases of bovine mastitis (Logan 1988). Some Bacillus species cause disease in insects, such as B. thuringiensis, which is widely exploited in agriculture as an insecticide by virtue of its plasmid-borne crystal toxin genes (Romeis et al. 2006), and B. larvae, recently reclassified Paenibacillus larvae, which causes American foulbrood in honeybees. ‘B. piliformis’ has been reclassified Clostridium piliforme on the basis of 16S rRNA sequence analysis and is responsible for Tyzzer’s disease (see Chapter 16) in laboratory mice and foals (Duncan et al. 1993).
Bacillus species
Natural Habitat
Pathogenesis and Pathogenicity
Anthrax
Genes
Virulence determinants
Functions
Plasmid pXO2
capB, capC and capA
Poly-D-glutamic acid capsule
Inhibits the phagocytosis of vegetative cells by macrophages
dep
Capsule degradation factor (dep)
A gene associated with the depolymerization of the capsular polymer: capsule degradation factor
acpA
Regulator of encapsulation (acpA)
A trans-acting regulatory gene: involved in the regulation of encapsulation
Plasmid pXO1
Lethal toxin (LeTx) made up of:
Triggers the oxidative burst pathway of macrophages and the release of cytokines (TNF-α and IL-1β)
lef
Lethal factor (LF)
Zinc-metalloprotease: cleaves various substrates and inactivates the mitogen-activated protein kinase kinase (MAPKK)
pagA
Protective antigen (PA)
Host-cell-binding moiety: facilitates the entry of LF into the host cell cytoplasm
Edema toxin (EdTx) made up of:
Increases cellular CAMP and disrupts water homeostasis
cya
Edema factor (EF)
Calmodulin-dependent adenylate cyclase
pag
Protective antigen (PA)
Host-cell-binding moiety: facilitates the entry of EF into the host cell cytoplasm
Others:
atxA
Activator of transcription (atxA)
Trans-acting regulatory genes: activates transcription of the anthrax toxin genes
pagR
pagR
Trans-acting regulatory genes
topA
topoisomerase (topA)
Type I topoisomerase
Sporulation process
Other bacilli
Bacillus species