Chapter 35 Avian Mycobacterial Disease
Mycobacteriosis is an ancient disease that continues to have a major impact in avian collections worldwide. Mycobacterial disease was “discovered” by Koch in 1882, although mycobacterial organisms have been isolated in human remains from 7000 bc and recovered from bison more than 18,000 years old.10,19,27 A Greek term, phthisis, was ascribed to tuberculosis-type lesions in 460 bc and, in the 1020s, a physician, Ibn Sina, in the Canon of Medicine, was the first to suggest that the disease caused by mycobacteria could be spread through contact with soil and water. It is these same characteristics that plague avian collections to this day.
By 1882, mycobacterial disease was reported in many avian species in zoological surveys. Reports included Darwin’s rhea, common fowl, common peafowl, golden pheasant, grouse, pigeon, partridge, stork, crane, falcon, and eagles.4 In 1886, Lehman and Neuman discovered what would be known as Mycobacterium avium, which was subsequently named based on its pathogenicity in poultry.12 In 1896, the term mycobacteria, or fungus bacterium, was introduced, with its name derived from the organism’s growth characteristics on liquid media.
Until 1990, most avian mycobacterial infections were attributed to M. avium (particularly serotypes 1 and 2). Around that time, however, a new species named M. genovense was isolated.4 It is also an opportunistic environmental mycobacterium, but has proven much more difficult to isolate using standard culture techniques, resulting in underreporting in many cases. Some less common mycobacterial isolates from birds include M. intracellulare, M. fortuitum, M. tuberculosis, M. gordonae, M. nonchromogenicin, M. bovis, and M. simiae.25
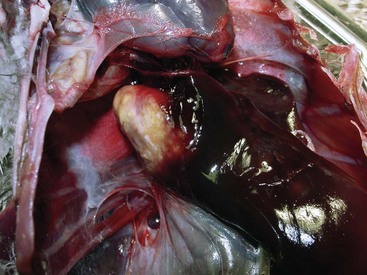
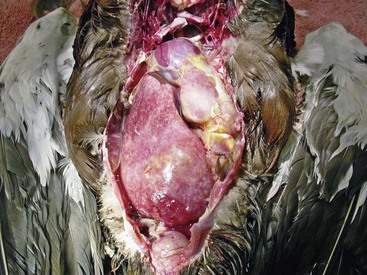
The term mycobacteriosis may apply to any disease resulting from a mycobacterial infection. It is often used when describing the nontubercle form of mycobacterial disease, a pattern typical of M. genovense infections. Similar lesions, as well as tubercle growth (avian tuberculosis; Fig. 35-1), or mixed-type lesions (Fig. 35-2) may occur with MAC infections. All lesion types are capable of shedding organisms.
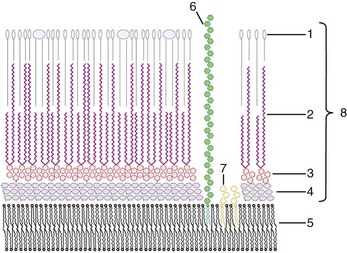
Structurally, the mycobacterial cell wall is an asymmetric membrane composed of a thin layer of tightly packed long-chain mycolic acids with a diverse array of free lipids (Fig. 35-3). The outer membrane is covalently linked to an arabinoside polysaccharide, which in turn is linked to an underlying layer of peptidoglycan.14 Additionally, glycopeptidolipids present in the cell wall are believed to be important for the formation of protective environmental biofilms.11 The high lipid content of the cell wall gives mycobacteria the ability to retain basic dyes in an acid-alcohol environment, leading to their acid-fast designation.
Species Affected and prevalence
Avian mycobacteriosis is a ubiquitous disease in domestic, captive, and wild birds. All birds appear to be susceptible, and no reports of totally resistant species exist. Although diagnosed more commonly in the north temperate zone, avian mycobacterial disease occurs worldwide. Observations by Hejlicek and Treml in 1995 regarding the relative susceptibility of avian species in large collections listed highly susceptible species as domestic fowl, sparrows, pheasants, partridges, and laughing gulls.9a Moderately susceptible species were turkeys and guinea fowl. Moderately resistant birds were reported to be geese and ducks. More highly mycobacteria-resistant species were thought to be pigeons, turtle doves, and rooks.
In general, the disease appears relatively uncommon in individual or small groups of captive held birds. A review of 5345 pet bird necropsies in Switzerland revealed a 3.8% incidence, with M. genovense infecting the majority.4 A recent survey of 23,960 necropsies of captive-held birds in the United States showed that mycobacteria was present in only 1.3% of the cases submitted.17
Disease prevalence is even lower, with notable exceptions, in free-ranging wild bird populations. Prevalence in free-ranging North American birds has been reported as less than 1%, which compares to a 0.7% prevalence in a study of 12,000 birds in the Netherlands.8 A review of 3000 U.S. wild waterfowl necropsies revealed a 0.3% infection rate.8 Mycobacterial infection in wild raptors is also considered rare and opportunistic.4 Individual flock history and environmental factors may play a major role in prevalence rates. A free-ranging U.S. whooping crane population was determined to have prevalence rates as high as 39% caused by specific environmental conditions favoring infection and spread.
Avian mycobacterial disease is most common in large flocks of zoological and avicultural collections, although marked prevalence variability occurs. Retrospective avian necropsy reviews from three U.S. zoological institutions have reported 1.2%, 4%, and 24.5% prevalence rates of mycobacterial disease, respectively.3,4,26 Husbandry protocols, environmental factors, and the species involved may have a dramatic impact on institutional incidence. In areas of environmental buildup, waterfowl and water birds, which are normally quite resistant in the wild, may be infected in large numbers.
Diagnosis and Immune Response
Hematologic changes most often occur late in the course of mycobacterial disease. When seen, nonspecific changes include heterophilia, monocytosis, hyperfibrinogenemia, thrombocytosis, polychromasia, and anemia.23 Marked hematologic changes in the form of significant heterophilic leukocytosis often signal the end stage of the disease process and, in some species, may be predictive of a terminal event.18 Potential serum chemistry alterations include increased hepatic enzyme and bile acid levels, as well as hyperproteinemia, hypoalbuminemia, and hyperglobulinemia. These changes, however, are unreliable and depend on the route of infection and stage of the disease.
Radiology may be effective to help substantiate a diagnosis of mycobacteriosis; however, it is also a nonspecific and inconsistent diagnostic tool. Signs may include organ enlargement, coelomic enlargement, pulmonary nodules and cystic bone lesions. Endoscopic and ultrasonic examinations may be helpful as well, but also suffer from low sensitivity and specificity, particularly in the early stages of infection.18
Traditionally, the gold standard for mycobacterial diagnosis has been culture. Culturing the organism, however, is often difficult, time-consuming, and dependent on a sufficient quantity of viable organisms being present (>100 organisms/mL).4 As a fecal screening test, cultures display low sensitivity and specificity because of environmental contamination issues and unpredictable organism shedding. Automated radiometric liquid culture techniques have improved isolation; however, culture still remains an unreliable screening technique on an individual bird basis.
Acid-fast staining is commonly used as a confirmatory test for swabs and tissues, but is also not an effective means of screening for disease. Accurate acid-fast staining is dependent on the absence of contamination and on the presence of more than 10,000 organisms/mL of sample. Acid-fast fecal screening in one study confirmed only 7% of culture-positive samples.4
Other modalities of organism detection have shown higher sensitivity and/or specificity for confirmatory testing, but are also not the answer for screening. DNA probes and polymerase chain reaction (PCR) testing, which may detect the equivalent of a single mycobacterial organism, are the preferred methods of antemortem and postmortem diagnosis of infected tissues and samples. PCR 16s-rRNA gene primers, adapted from M. bovis tests, have been very effective in identifying M. avium samples.4 PCR testing has been further used to help differentiate virulent strains using primers such as IS901 insertion sequences.16 Fecal screening techniques involving PCR technology are hampered by the common issue of unreliable organism shedding. Also, PCR fecal inhibitors and environmental contaminants may negatively affect accuracy. Newer generations of real-time PCR assays, including those targeting 65-kDa heat shock proteins, are being used for improved sample diagnosis. Real-time PCR testing also has demonstrated improved capabilities for quantitative results, with reduced risk of contamination and less sensitivity to inhibitors.
Response to mycobacterial infection is initially controlled by cell-mediated mechanisms but, unfortunately, mycobacteria have evolved many means to evade elimination by the host. Mycobacteria are able to suppress host macrophage production selectively and can block destruction by preventing the normal fusion of phagosomes with lysosomes. Mycobacteria may hide, survive, and even replicate in the host macrophages for years, only to develop into an active infection when the host’s immune system is suppressed. Cell-mediated testing, such as the intradermal skin test, used effectively in the commercial poultry industry for flock screening, is unreliable in nondomestic species.15
Humoral systems do not seem to play a major protective role and become demonstrable only in the later stages of disease. As noted, humoral responses differ among bird species and display no pathognomonic patterns. Serologic tests such as rapid agglutination testing, enzyme-linked immunosorbent assay (ELISA), and Western blot tests have been used with varying success for flock diagnosis. Western blot analysis, as an example, displayed a sensitivity of 88.24% and specificity of 100% in one study of a known infected population of ring-necked doves.9 However, results did not correlate with disease severity or stage of infection.
ELISA’s have also been developed for use in specific avian populations. Commercial tests are generally unavailable for most exotic species, and the requirement for species-specific antigens makes widespread use of these tests difficult and costly for large mixed collections. To date, ELISA’s have not shown an adequate level of sensitivity and specificity in initial disease stages to serve as quarantine screening tests, although specific ELISA’s have been used effectively for later stage culling in infected populations.4 With all serologic testing, best results are seen with flock screening rather than use on the individual level.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree