Chapter 25 Avian Atherosclerosis
Vascular pathology in avian species includes a spectrum of lesions and etiologies similar to conditions in mammals. Of these conditions, atherosclerosis is the most common vascular change across the class Aves.
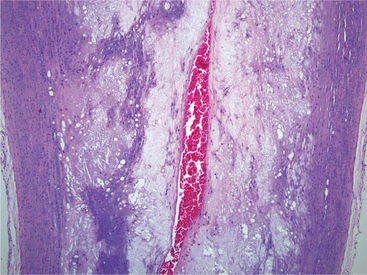
Arteriosclerosis is a loss of arterial elasticity caused by intimal thickening. This thickening results from migration of smooth muscle cells from the tunica media and increased mitotic division of these cells. Intimal expansion occurs from elaboration of increased extracellular matrix (ECM).18 Arteriosclerotic plaques of varying size, composed primarily of fibrous tissue between the intima and internal elastic lamina, are common findings in many birds. These plaques can form in chickens as young as 4 weeks of age. The plaques are appreciated grossly as pale areas of increased thickening of the intima, most often in the aorta and major arteries.15 These plaques may extend as circumferential lesions with marked luminal narrowing (Figure 25-1).
Atherosclerotic plaques are composed of collagen and proteoglycans. Smooth muscle cells produce the ECM of the plaques. After 6 weeks of a cholesterol-supplemented diet, Japanese quail with a propensity to develop atherosclerosis have elevated glycosaminoglycan levels and foam cell development in atheromatous plaques. These foam cells disrupt the collagen ECM. After 10 weeks on a cholesterol-supplemented diet, there is disorganization in aortic intimal collagen fibrils. Total vascular collagen does not necessarily increase.25
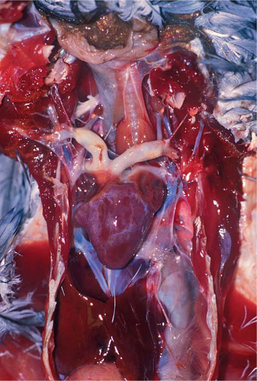
Atherosclerotic lesions in birds occur at the great vessels at the base of the heart and within the heart itself (Figure 25-2). The most frequently affected site is the aorta at the heart’s base. Other sites of importance include the brachiocephalic trunk, pulmonary artery, dorsal aorta, heart valves, and mural arteries. Aortic atherosclerosis in penguins may variably extend along the aorta to the level of the renal arteries. In all cases, lesions are more pronounced at the level of, or just before, the branching of smaller arteries. Unlike the condition in humans, associated aneurysmal dilation is not common in birds, except turkeys.
Turkeys are also the exception that makes the rule for lesion distribution of this condition. The muscular abdominal aorta of turkeys is much more susceptible to atherosclerosis than the elastic thoracic portion. In a study of 157 wild male turkeys collected by hunters in the early 1980s, birds demonstrated lesions most frequently in the sciatic arteries. Other affected sites, in order of decreasing frequency, were the aorta at the celiac region, cranial abdominal aorta, aorta at the sciatic bifurcation, caudal abdominal aorta, coronary arteries, and finally, thoracic aorta.17 This dramatically different lesion distribution suggests a possibly different etiology compared with other avian species. In quail, etiologic conditions respon-sible for atherosclerosis of the abdominal aorta are different than those resulting in lesions in the coronary arteries.
Relationships of atherosclerosis to age and gender in birds are not as clear-cut as in humans. Atherosclerosis is generally a condition of adults. In spontaneous avian atherosclerosis, although lesions have been reported in a bird less than 1 year of age, most affected birds are older adults, with severity increasing with age.2 Gender predilections have varied from report to report. Some studies demonstrate a male bias,11 some a female bias,10 and some no gender bias at all.2 In a review of 57 cases of atherosclerosis in seven species of penguins at all SeaWorld parks, there was no gender predilection. Lesions were limited to animals older than 4 years, and severity did increase with increasing age within species.
CLINICAL SIGNS
Vascular conditions associated with atherosclerosis include vascular rupture in penguins24 and a vulture.11 The condition in penguins was sometimes associated with prodromal signs of anorexia and decreased responsiveness to keepers on the morning preceding death. Sudden death occurred with no clinical signs in most cases. Cardiac disease identified on necropsy suggests that dyspnea and exercise intolerance may have been present as unidentified clinical conditions.
Atherosclerosis in turkeys and polar penguins has been associated with aortic rupture.15,24 In these cases the rupture is likely secondary to the vascular wall degeneration associated with atheromatous changes. In turkeys, aortic rupture may cause mortality rates up to 20% in male bird flocks. These birds demonstrate atherosclerosis, aneurysmal dilation, and rupture. Aortic rupture in polar penguins was also seen in areas without distinct atheromatous degeneration. The similarities in aortic rupture between these species are interesting. Both species typically demonstrate atheromatous plaques. Areas of aortic rupture are more likely abdominal in turkeys and at the heart base in polar penguins.
Atherosclerosis has also been associated with clinical signs in multiple organ systems because of reduced blood flow to critical organs. Most clinical signs are referable to poor blood supply to the brain or muscles or to secondary cardiopulmonary disease.2 Vascular luminal stenosis related to atherosclerosis in multiple vessels in a 16-year-old cockatoo resulted in clinical lethargy, decreased appetite, and falling off the perch. Clinical signs in multiple species include lethargy, disorientation, seizures, fainting, dyspnea, anorexia, regurgitation, and leg lameness to paralysis. Extensive atherosclerosis in the sciatic arteries of wild turkeys17 and at the origin of the celiac arteries in white Carneau pigeons7 suggests that examination of the abdominal aorta and its branches may identify causes for leg lameness previously undiagnosed in cases of significant atherosclerosis.
DIAGNOSIS
Multiple studies have attempted to correlate atherosclerosis with changes in serum cholesterol and lipoprotein levels. In humans the relationship between elevated serum cholesterol and atherosclerosis is well known. In pigeons, similar serum cholesterol levels were found in atherosclerotic-prone birds and nonprone birds between 3 and 12 weeks of age, despite atheromatous lesions being present in 13 of 33 birds on examination at 12 weeks of age.7 However, other studies have contradicted this; Bavelaar2 reported correlations between plasma cholesterol levels and severity of atherosclerosis in chickens, quail, and pigeons, suggesting an association of intrinsically high cholesterol with species predisposed to atherosclerosis.
Electrocardiograms (ECGs) are becoming increasingly common in avian diagnostics. Normal findings are available for a variety of avian species, including peregrine falcons, psittacines, and gulls.16,18,20,21 Normal ECG values have been published for groups of anesthetized and unanesthetized patients. Anesthesia may improve positioning of the patient and decrease muscular trembling. Conversely, some have reported anesthesia-associated arrhythmias.4,26 Because atherosclerotic changes often preferentially affect the aorta, causing luminal constriction and impeding flow, left ventricular enlargement with associated ECG changes is a possible clinical finding. ECG changes associated with left ventricular hypertrophy in humans include an increase in the QRS amplitude and prominent septal Q waves.5 Cardiac rhythm abnormalities are associated with atherosclerosis in humans.
Necropsy examination for detection of incidental and clinical atherosclerosis requires a systematic approach. Cardiovascular examinations should include opening cardiac chambers, examining the myocardium and heart valves, and extending incisions to major vessels to examine the intimal surface of vessels at the heart’s base. Examination of the aorta should extend from the heart base along the thoracic and abdominal aorta to the level of the celiac arteries. Multiple arteries may be affected, and histologic changes in myocardial, splenic, gastric, and meningeal vessels may be seen only if these organs are examined histologically. Arteriosclerotic and atherosclerotic changes may vary from minor, firm thickenings of the tunica intima to circumferential, hard thickenings of vessels with extensive luminal stenosis. Samples of the affected areas should be collected in 10% neutral buffered formalin and processed routinely for histologic review.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree