Chapter 41 Avian Analgesia
Recognizing Pain in Birds
Behavioral changes may be very cryptic and subtle, but are often the earliest signs of pain detected by animal care staff or owners. Behavioral changes are often species-specific, and observers must be familiar with the full range of normal behaviors for the species as well as the individual. It is important to observe birds at appropriate times for each species, such as observing nocturnal species at night. Certain behaviors have evolved and have survival value; for example, immobility is a common behavior displayed by birds when being observed or examined, making pain evaluation challenging. In chickens, immobility has also been associated with prolonged pain, stress, and fear responses. Feather grooming is an avian behavior that may express the full spectrum of change associated with pain, both acute and chronic. Grooming activity may decrease when a bird is in pain or, conversely, overgrooming and feather destructive behaviors have been associated with chronic pain. In a social species housed with a group, a painful bird frequently isolates itself and may sleep apart from the rest of the flock, and mutual grooming is often decreased. In some species, a display of discomfort, illness, or weakness may gain support or protection from conspecifics, but similar behavior in another species may attract attention from predators or lower its status in the flock’s social order. Increased grooming activities to themselves or other birds may also be an intentional distraction. Studies using chickens with experimental arthritis have demonstrated that shifting attention may reduce painful behaviors and potentially reduce peripheral inflammation.9
Pain occurs along a gradient and, in lieu of species-specific pain score sheets for birds, there is tremendous value in using a generic pain scale of 1 to 10 to evaluate a bird’s pain and response to treatment. In one study, pigeons following orthopedic surgery were evaluated using a detailed numeric rating scale plus a simple 1 to 10 pain scale, and there was significant correlation between both methods.23
Evaluation of Analgesics
To determine the efficacy of an analgesic in any species, it is important to determine the pharmacokinetics (PK) and pharmacodynamics (PD) of the drug in that species. Integrating PK and PD data provides a basis for selecting clinically relevant dosing schedules. When the same dose produces different plasma concentrations in different species of birds, the variance is caused by PK. When the same plasma concentration produces different responses in different species, the variance is caused by PD. PK studies of analgesic drugs are insufficient to determine appropriate doses and dosing frequencies because plasma concentrations of opioids and nonsteroidal anti-inflammatory drugs (NSAIDs) do not always correlate with delivery of analgesia. Plasma concentrations provide guidance for dosing frequencies, but it has been shown that the duration of the analgesic effect of NSAIDs may be longer than that predicted from plasma levels. The PK of analgesics varies considerably across all species that have been studied, so extrapolating clinical doses and dosing intervals from one species to another species is not appropriate. To date, very few PK studies have been published for analgesics in birds (Tables 41-1 and 41-2).
TABLE 41-1 Opioid Analgesics Evaluated in Avian Species by Pharmacokinetic (PK) or Pharmacodynamic (PD) Studies
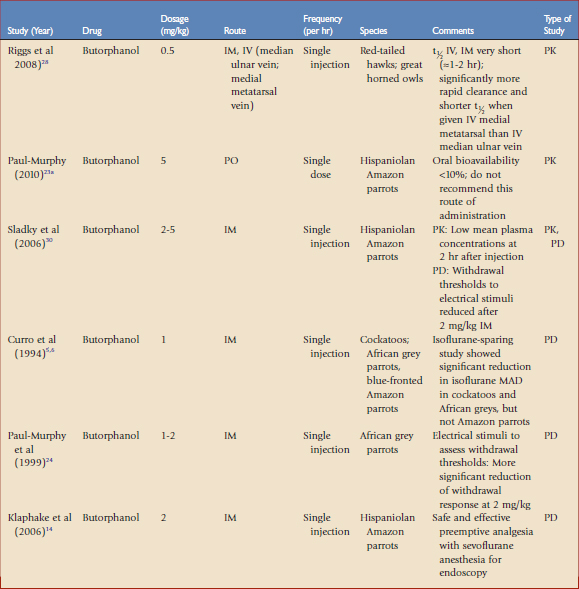
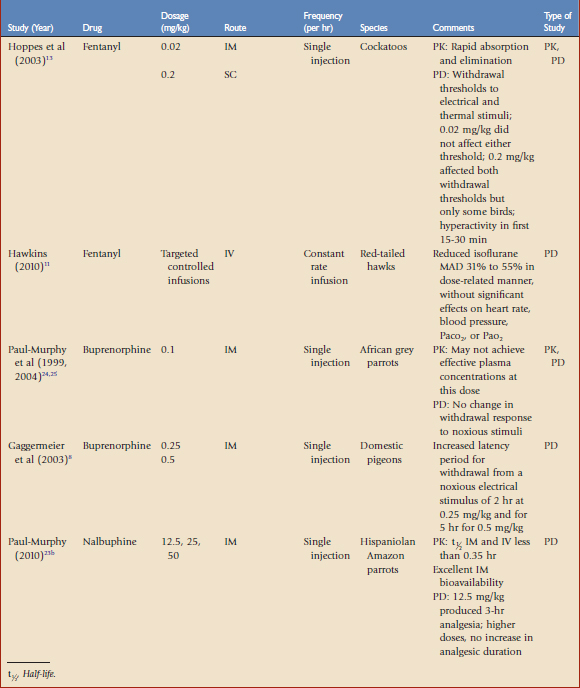
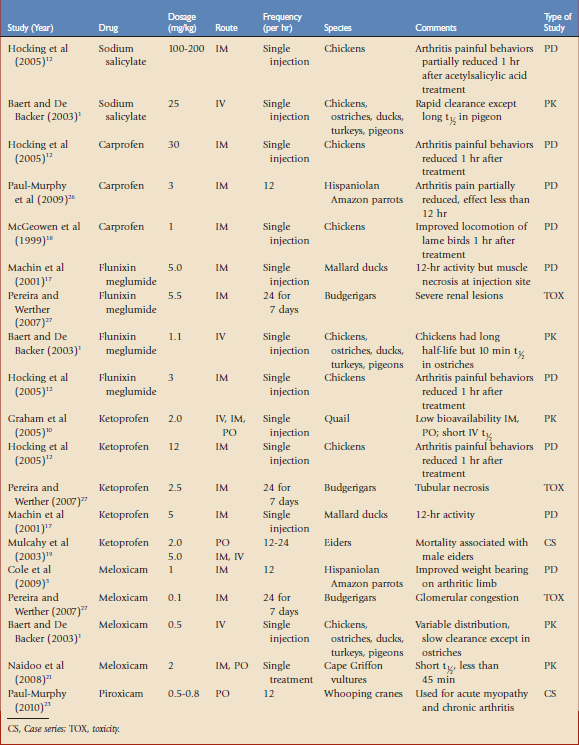
TABLE 41-2 Nonsteroidal Anti-Inflammatory Drugs Evaluated in Avian Species by Pharmacokinetic (PK) or Pharmacodynamic (PD) Evaluations, or Toxicologic (TOX) or Clinical Studies
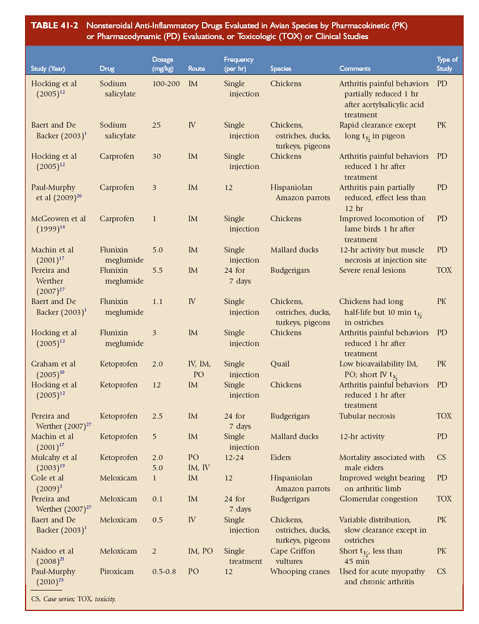
Inflammatory models are used to create painful conditions with repeatable behaviors or weight bearing that may be quantified, such as acute synovitis-arthritis models in chickens and parrots.12 In one model, the injection of urate microcrystals into the intertarsal joint caused acute inflammatory arthritis, leading to measurable changes in weight bearing and lameness. A study with Hispaniolan Amazon parrots (Amazonia ventralis) differentiated significant weight-bearing improvement when birds were given butorphanol versus less improvement in the same birds given carprofen.26 Evaluating the response of a bird to a noxious stimulus with and without analgesics is used to determine efficacy when thresholds may be measured. The noxious stimuli used in avian analgesia studies are variable, but usually include withdrawal thresholds to thermal, electrical, or pressure stimuli. Experimental models have been developed in chickens and parrots, but these models may not extrapolate to pain behaviors relevant to clinical pain. Therefore, the doses and dosing frequencies recommended in the published reports should always be critically evaluated on a case by case basis.
Opioids
Opioids are used for moderate to severe pain, such as traumatic or surgical pain. Opioids reversibly bind to specific receptors in the central and peripheral nervous system. These drugs are categorized as agonists, partial agonists, mixed agonists-antagonists, or antagonists based on their ability to induce an analgesic response once bound to a specific receptor. The agonist drugs have a linear dose-response curve that may be titrated to reach the desired effect, whereas the agonist-antagonist drugs may reach a ceiling effect, after which increasing the dose does not appear to provide additional analgesia. During anesthesia, opioids are used to provide perioperative analgesia that may reduce the concentrations of volatile anesthetics (e.g., gas anesthesia–sparing effects). The most common adverse effects reported with opioids are cardiac and/or respiratory depression. In many cases, these drugs may be reversed with antagonists, which will also terminate analgesia. The application and dosages of several opioid formulations have been scientifically evaluated and clinically applied in birds (see Table 41-1).
Most opioid analgesics are used parenterally because of poor oral bioavailability associated with the first-pass effect. Once absorbed, oral opioids first pass through the liver, where they are metabolized, releasing a significantly lower amount of active drug into the general circulation. For example, the bioavailability of 5 mg/kg orally administered butorphanol in Hispaniolan Amazon parrots was lower than 10%, making this route ineffective.23a
Opioids vary in their receptor specificity and efficacy in mammals, which results in a wide variety of clinical effects in different species. It is reasonable to presume that this opioid variability will also have a wide range of clinical effects in avian species. The distribution of opiate receptor types is well conserved across all mammalian species in the brainstem and spinal cord, but may vary markedly in the forebrain and midbrain. There is an overall lack of published data concerning differences in opioid receptor distribution, density, and functionality in birds. However, early work showed that the regional distribution of mu, kappa, and delta receptors in the forebrain and midbrain of pigeons were similar to mammals, but 76% of opiate receptors in the forebrain were of the kappa type. Kappa receptors have multiple physiologic functions in the bird and the analgesic function of these receptors still needs further investigation. It has been postulated that this difference in receptor distribution and density may partially explain why some avian species do not appear to respond to mu agonists in the same manner as mammals. However, in day-old chicks, marked dissimilarities to this distribution suggest either age- or species-related differences. It has been postulated that birds may not possess distinct mu and kappa receptors or that the receptors may have similar functions. This may explain in part why the isoflurane-sparing effects of mu and kappa agonists in chickens appear to be similar to those in mammals.4
Fentanyl
Fentanyl is a short-acting mu receptor agonist that has not been used commonly in avian medicine because historical investigations with morphine (the standard for mu opioids) administered to chickens were confusing and clinically inconclusive. Fentanyl, 0.02 mg/kg IM, did not affect the withdrawal thresholds to electrical or thermal stimuli of white cockatoos; a tenfold increase in the dosage (0.2 mg/kg SC) did produce an analgesic response, but many birds were hyperactive for the first 15 to 30 minutes after receiving the high dose.13 Fentanyl had rapid absorption and elimination in parrots, with mean residence times of less than 2 hours (see Table 41-1). Because of its short-acting properties, fentanyl delivered via constant rate infusion (CRI) is an excellent choice as an analgesic adjunct to inhalant anesthesia in mammals and, when used at low doses as a CRI, we have found fentanyl may also be effectively used in avian anesthetic protocols. Fentanyl administered as an IV CRI in red-tailed hawks (Buteo jamaicensis) to target plasma concentrations of 8 to 32 ng/mL reduced the MAD of isoflurane 31% to 55% in a dose-related manner, without statistically significant effects on heart rate, blood pressure, Paco2, or Pao2.11 Fentanyl may also be combined with ketamine as a CRI, thereby reducing the dosages of each that are needed.
Butorphanol
Butorphanol is a mixed agonist-antagonist with low intrinsic activity at the mu receptor and strong agonist activity at the kappa receptor. Adverse effects associated with butorphanol such as dysphoria have not been reported in birds. Preoperative butorphanol administration (2 mg/kg IM) did not show significant anesthetic (including time to intubation and extubation) or cardiopulmonary changes in Hispaniolan Amazon parrots anesthetized with sevoflurane, suggesting that it may be useful as part of a preemptive analgesic protocol (see Table 41-1).14 Earlier isoflurane-sparing studies using 1 mg/kg IM butorphanol in cockatoos, African grey parrots (Psittacus erithacus erithacus), and blue-fronted Amazon parrots (Amazona aestiva) showed a significant MAD reduction in the cockatoos and African grey parrots, but not in the blue-fronted Amazon parrots.5,6 In a study using withdrawal thresholds to electrical stimuli in conscious African grey parrots, butorphanol, 1 to 2 mg/kg showed a decreased withdrawal effect that was more significant at 2 mg/kg.24 Butorphanol dosages of 3 to 6 mg/kg IM had similar analgesic effects on Hispaniolan Amazon parrots. Based on these studies, dosages of 1 to 4 mg/kg have been suggested in birds. A recent study evaluating the PK of 0.5 mg/kg butorphanol in red-tailed hawks (Buteo jamaicensis) and great-horned owls (Bubo virginianus) found half-lives of 0.93 and 1.78 hours, respectively, when given IV and 0.94 and 1.84 hours, respectively, when given IM.28 Similarly, low serum butorphanol concentrations were evident in Hispaniolan Amazon parrots 2 hours after a single IM administration of a 5-mg/kg dose.30
These data suggest that frequent dosing of butorphanol may be necessary in birds. This frequency of dosing is, in some cases, impractical because of lack of personnel to provide frequent dosing and the stress of frequent handling on the patient. A liposome-encapsulated, long-acting form of butorphanol tartrate was shown to be safe and effective in Hispaniolan Amazon parrots for up to 5 days following SC administration,30 and was also shown to be an effective analgesic in Hispaniolan Amazon parrots with induced arthritis (see Table 41-1).26 The results from these studies are encouraging because a long-acting formulation of butorphanol would allow for both reduced frequency in butorphanol dosing and handling of avian patients for drug administration. Unfortunately, this formulation is not yet commercially available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


