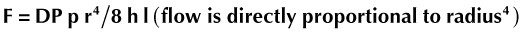CHAPTER 44 Asthma
PATHOPHYSIOLOGY
EOSINOPHIL AND T-LYMPHOCYTE INTERACTIONS
The pathogenesis of asthmatic airway hyperreactivity clearly is multifactorial. Numerous investigations suggest that the interaction between T lymphocytes and eosinophils within airways may play an important role in the generation of airway inflammation and airway hyperreactivity in human asthma.1–3 Increased numbers of eosinophils and activated T lymphocytes are found in bronchoalveolar lavage specimens and bronchial mucosa of patients with asthma. The presence of these cells is correlated with disease severity. Activated T cells are recruited into the airways of asthmatic individuals when they are exposed to aeroallergens. These CD4-positive lymphocytes (among other cells) secrete interleukin-5 (IL-5) to promote eosinophilopoiesis, survival, activation, and recruitment into airways. A T helper 2–driven cytokine profile has been demonstrated in antigen-induced asthma models in the feline species.4
STUDIES IN NATURALLY OCCURRING DISEASE
Although coughing and wheezing cats have been identified by owners and veterinarians for almost a century, only in the last 15 years have we begun to study the disorder in earnest. Dye and associates identified pulmonary function abnormalities in cats with signs of chronic lower airway inflammation.5 Some of these cats had increased pulmonary resistance that resolved after treatment with terbutaline, a β2-agonist, indicating the presence of reversible bronchoconstriction in these patients. Additionally, some of these cats experience dramatic bronchoconstriction after exposure to low levels of methacholine, a drug with minimal effects on pulmonary function when used in equivalent doses in nonasthmatic cats. This was the first demonstration of spontaneous, naturally occurring airway hyperreactivity and reversible bronchoconstriction in a nonhuman species. Additionally, histological changes in airways from asthmatic cats include epithelial erosion, goblet cell and submucosal gland hyperplasia and hypertrophy, and an increased mass of smooth muscle, which are features of human asthmatic airways. Additional reviews have demonstrated the variation in clinical findings, radiographic patterns, and responses to therapy in cats with bronchial disease.6,7 This likely is due to differences in staging these disorders, and the fact that other disorders, including pulmonary fibrosis and heartworm infection, can mimic the signs of bronchial disease in cats.8,9
EXPERIMENTALLY INDUCED FELINE ASTHMA
Experimental models of feline asthma have been developed to better understand the immunological mechanisms operative in the pathogenesis and perturbation of asthma and as a means to determine responses to therapy objectively. The first model of feline asthma involved antigen sensitization and chronic aerosol challenge with Ascaris suum as an antigen. These cats developed persistent airway eosinophilia and hyperresponsiveness to nebulized acetylcholine, with typical morphological changes observed in spontaneous bronchial disease of cats. These antigen-sensitized cats had elevated serum levels of soluble receptor for IL-2 found within 24 hours of antigen challenge and a decrease in the CD4+:CD8+ peripheral blood T-cell ratio compared to control values. These findings suggest T-cell activation in asthmatic cats similar to the findings in human beings with the disease.10 In this experimental model, we found that treatment with cyclosporine A (CsA) dramatically inhibited the pathological changes in airway structure and function seen in cats not treated with CsA.11 However, these findings should not be interpreted to mean that cyclosporine should be used to treat naturally occurring asthma in cats (see section on Treatment). The author has shown that serotonin is a primary mediator in feline mast cells that contribute to airway smooth muscle contraction in vitro.12 This mediator is absent in human, equine, and canine airways. During an acute asthmatic attack, inhaled antigens within airways promote mast-cell degranulation acutely with release of preformed serotonin. These mediators cause sudden contraction of airway smooth muscle. Interestingly, it has long been assumed that histamine released from feline mast cells caused acute bronchoconstriction. This assumption has been challenged recently by the finding that histamine nebulized into cat airways has an unpredictable effect from one cat to another. Specifically, histamine may have no effect, it may cause bronchoconstriction, or it may actually dilate feline airways.
Norris Reinero et al developed an experimental model in which cats were sensitized and challenged chronically with either Bermuda grass allergen (BGA) or house dust mite to mimic well-recognized antigenic triggers of human asthma. These cats produce allergen-specific immunoglobin E (IgE), allergen-specific serum and bronchoalveolar lavage fluid (BALF) IgG and IgA, airway hyperreactivity, airway eosinophilia, an acute T helper 2 cytokine profile in peripheral blood mononuclear cells and BALF cells, and histological evidence of airway remodeling.4 This model has been particularly helpful in evaluating specific immunomodulating treatment strategies. For example, cats sensitized and challenged with BGA to develop the asthmatic phenotype were treated with rush immunotherapy (parenteral high doses of BGA). These cats had significantly reduced eosinophil counts.13 More recently, Reinero et al used CpG motifs (microbial oligodeoxynucleotide products that modulate activity in human and murine lymphocytes) in cats with BGA-induced airway inflammation and hyperreactivity to show that this approach dampened the eosinophilic response normally seen in these antigen-sensitized and challenged cats; however, the hyperactivity within airways was not affected.14
CLINICAL FINDINGS IN FELINE ASTHMA
INCIDENCE AND PREVALENCE
Currently, there are no reliable data regarding the incidence and prevalence of asthma in cats. The prevalence of lower airway disease in the general adult cat population is estimated to be approximately 1 per cent; prevalence in the Siamese breed may be 5 per cent or greater.15 In 2000, a website (http://www.fritzthebrave.com) was developed to draw attention to the diagnosis and treatment of feline asthma. One result of this website was the generation of a “list-serve” with greater than 500 members, each of whom is the owner of one or more cats with asthma. A poll of these members revealed that more than 15 per cent of the owners represented by this relatively random sample had Siamese cats (Kathryn Hopper, personal communication).
DIAGNOSIS
THORACIC RADIOGRAPHS
Radiographs of the thorax of cats with asthma commonly demonstrate bronchial wall thickening, usually described as “doughnuts” and “tramlines” (Figure 44-1). Air trapping may be evidenced by hyperinflated airways. This is seen most prominently on the lateral view and can be appreciated by recognizing the position of the diaphragmatic crus at approximately the level of L1-L2.
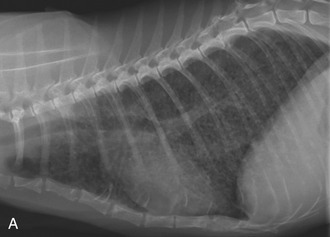
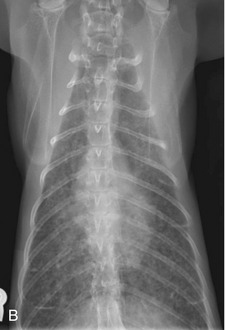
In the author’s experience, approximately 15 per cent of thoracic radiographs of cats with bronchial disease have increased density within the right middle lung lobe associated with a mediastinal shift to the right. This is evidence of atelectasis. It usually is easier to see this pattern on a dorsoventral or ventrodorsal exposure because the right middle lung lobe silhouettes with the cardiac silhouette on the lateral view (Figure 44-2). Atelectasis occurs most commonly in the right middle lung lobe because of mucus accumulation within the bronchus, and this airway is involved most frequently because it is the only airway that has a dorsal/ventral orientation within the bronchial tree and therefore subject to the effects of gravity. In more extreme cases, the clinician may appreciate fluffy, ill-defined heavy infiltrates in multiple lung lobes. The cause of these changes in cats with lower airway disease may be many small areas of atelectasis in several lung lobes resulting from multiple diffuse small mucus plugs. This presents a diagnostic challenge because this radiographic change is consistent with a number of disorders, including neoplasia and diffuse interstitial pneumonitis. Importantly, routine survey thoracic radiographs may be normal and should not cause the practitioner to abandon the diagnosis of asthma.
BRONCHOSCOPY
The author emphasizes that bronchoscopy is rarely required to make an accurate diagnosis of asthma in feline patients. Bronchoscopy in healthy cats is not a trivial undertaking. In cats with cough and respiratory compromise, bronchoscopy may be a life-threatening procedure16 and only should be performed by persons adequately and formally trained in the technique (see Chapter 45). Instead, the previously described historical and physical examination findings and results of fecal and heartworm testing and thoracic radiography are sufficient to make a presumptive diagnosis. Definitive diagnosis usually can be made in these patients by a strongly positive response to therapy. In fact, in the author’s experience, the primary indication for bronchoscopy in these patients occurs when there is not an otherwise predictable cessation or minimization of clinical signs after 6 to 7 days of aggressive corticosteroid treatment. Some clinicians prefer to obtain airway washings and culture before initiation of long-term respiratory therapy, although these points made here and in the next section should be considered before initiating bronchoscopy and interpreting the cytological and culture results.
TRACHEOBRONCHIAL CYTOLOGY
Asthmatic cats commonly have clinicopathological evidence of airway inflammation, including the finding of large numbers of eosinophils recovered from bronchial secretions. Until the 1980s, it generally was assumed that eosinophils played only a beneficial role in the immune system by protecting against parasite infestation. Within the last 20 years, however, it has become clear that the presence of these cells in the wrong place at the wrong time can result in significant cellular and tissue damage. It is therefore of great interest that eosinophils (often 20 to 25 per cent of total count) can be recovered in large numbers from the tracheobronchial washings of many healthy cats.17 These cells appear to cause no damage to the local tissue environment, and their presence should not be assumed to indicate allergy or parasitism. For this reason, airway lavage fluid eosinophilia should not be used to diagnose asthma definitively in the feline species. Similarly, alveolar macrophages are a normal cell within the lung parenchyma and are the most common cell recovered from BALF obtained from healthy cats. These cells do not represent “granulomatous” or “histiocytic” inflammation reliably when obtained in airway lavage fluid from asthmatic cats.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree