Chapter 32 ARTIFICIAL INSEMINATION, FRESH EXTENDED SEMEN, AND FROZEN SEMEN
The manual collection and subsequent deposition of semen into the vaginal vault of a bitch in estrus (standing heat) is a common procedure used by breeders and veterinarians. As a result, artificial insemination (AI) is frequently requested by the dog owner or handler. Fresh undiluted semen, semen mixed with an extender, or frozen semen is used in dogs. The chances of success are enhanced if the veterinarian has a good understanding of the estrus cycle, semen collection, and AI techniques, as well as potential pitfalls.
INDICATIONS FOR ARTIFICIAL INSEMINATION
Some owners wish to use AI to avoid any possible venereal contact between their dog and its mate, thereby controlling the spread of potentially infectious diseases. This is not sound reasoning when the concern is the transmission of infectious agents from the dog to the bitch. Inseminating semen into the vagina still provides intimate contact between bitch and stud dog. Any infectious agent that could be transmitted from the dog to the bitch during natural mating also has the potential to be transmitted during AI. However, AI does avoid transmission of infectious agents from the bitch to the stud dog. All breeding dogs and bitches should be free of Brucella infection, as determined by appropriate tests (see Chapter 26).
Some male dogs (particularly Doberman Pinschers) experience prostatic bleeding and hemospermia after exposure to a bitch “in heat” (estrus; see page 1000). The bleeding may be associated with von Willebrand’s disease (VWD) but has also been observed in dogs not afflicted with VWD. Regardless, AI can be performed without any contact between stud dog and bitch, occasionally avoiding this problem.
ARTIFICIAL INSEMINATION USING FRESH SEMEN
Collection of Semen
Semen is usually collected in an artificial vagina. We cut off and use one end of a sterilized, soft rubber, cone-shaped bovine artificial vagina. To this is attached a 12 to 15 ml plastic tube (Fig. 32-1; also see Fig. 27-3, page 934). The wide-mouth end of the cone is folded over and sealed with rubber cement to make a smooth, nonabrasive edge. A small amount of nonspermicidal lubricating jelly is applied to the inner surface of the rubber cone. This is the only surface that makes penile contact.
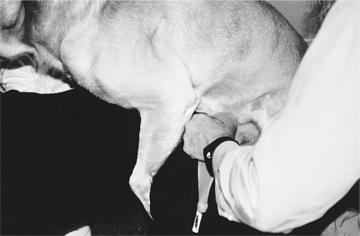
The most difficult task in AI is stimulating the male to ejaculate. Once this has been accomplished, the balance of the procedure is quite simple. With most stud dogs, semen can be collected in a clean, quiet room with a nonslippery floor. A bitch is not often needed. However, for experienced males accustomed to natural breeding, a bitch in heat makes collecting semen easier and may improve the quality of the semen sample collected. Application of a topical pheromone preparation (Eau d’Estrus; Synbiotics, Malvern, PA) to a nonestrual bitch may also help. With the owner holding the stud dog to minimize movement and to protect the collector, the penis and bulbus glandis are gently massaged within the penile sheath. When the bulbus glandis begins to enlarge, the sheath is slipped posteriorly, and the penis with the bulbus glandis is exteriorized (Fig. 32-2). Failure to exteriorize both the penis and the bulbus glandis from the sheath usually results in an incomplete erection and failure to ejaculate or incomplete ejaculation, presumably due to pain.
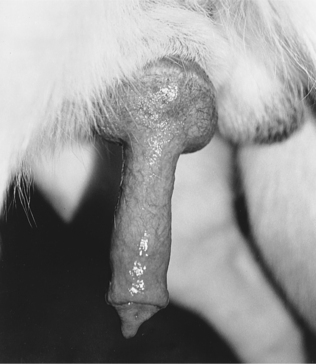
FIGURE 32-2 Erect penis of a dog with an engorged bulbus glandis and prepuce pulled posterior to the bulbus glandis.
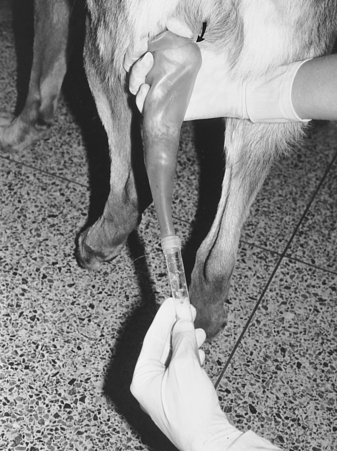
Once the penis and bulbus glandis have been extruded from the sheath, the collector firmly holds onto the base of the penis, proximal to the bulbus glandis (Fig. 32-3). The thumb and index finger are used, providing both massaging movements and downward pressure around the base of the bulbus glandis. During or immediately after an erection has been achieved, aggressive pelvic thrusting movements by the stud dog, which accompany the onset of ejaculation, may make it difficult to place the artificial vagina over the penis. Fortunately, pelvic thrusting is typically short-lived (5 to 30 seconds), the initial phase of the ejaculate consists primarily of sperm-free prostatic fluid, and the sperm-rich second fraction of the ejaculate usually begins as the pelvic thrusting begins to subside. Therefore there should be no alarm over failure to collect ejaculate produced at the beginning of pelvic thrusting. Immediately after pelvic thrusting stops, many males try to “step over” the collector’s arm, as if dismounting the bitch. The collector should simply allow this movement by the male, which results in a 180-degree rotation of the engorged penis such that it is protruding caudally between the rear legs (Fig. 32-4). Digital pressure should be maintained on the bulbus glandis and collection continued until the ejaculate becomes clear (see Fig. 27-4, page 934).
Semen is usually collected for 2 to 5 minutes. This represents the typical duration of the second (sperm-rich) phase of ejaculation. The clear plastic tube should already have been connected to the rubber artificial vagina, and the apparatus can be held under the collector’s arm during the initial stimulation period to provide some warmth. Canine semen is relatively resistant to cold shock, which alleviates the need for warm water baths or incubators for holding semen. Use of a clear plastic collection tube allows visualization of the semen. One hand is kept over the plastic to avoid excessive light exposure. As long as the ejaculate is obviously whitish or creamy and cloudy (normal and sperm rich), the ejaculate continues to be collected. When the ejaculate becomes clear (sperm free), collection can be discontinued. If the collector is not certain when the male has stopped ejaculating the sperm-rich fraction, collection should be stopped after 5 minutes. Continued collection only dilutes the sperm with the third-phase clear, sperm-free prostatic fluid, resulting in cumbersome fluid volumes.
Insemination Procedure
VAGINAL INSEMINATION USING A URINARY CATHETER OR INSEMINATION PIPETTE.
Although a variety of “tools” are available for vaginal insemination, we routinely use a 12 ml syringe, flexible disposable male urinary catheter or rigid plastic insemination pipette (Synbiotics; Malvern, PA), and surgical gloves. The catheter or insemination pipette should be long enough to reach the cranial vagina, which can be estimated as half the length from the vulvar lips to the costal arch (Root Kustritz and Johnston, 2000). These items should be sterile. After the gloves have been put on, the semen sample is drawn into the syringe, the sterile catheter is attached, and the syringe is filled with an additional 1 to 3 ml of air. The bitch’s rear end is elevated above her head during and immediately after completion of the insemination. Usually we have owners sit in a chair and place a drape over the lap, and then hold the rear legs of the bitch in the lap (Fig. 32-5). With a technician holding the tail to the side, a gloved, nonlubricated index finger (except in very small dogs) is placed into the vaginal vault, palm up. If a lubricant is used, it must be nonspermicidal. The catheter is slid over the top of the finger and passed into the vaginal vault, avoiding accidental catheterization of the urethra. Sliding the catheter over the index finger also aids in avoiding the clitoral fossa. The catheter follows the dorsal curvature of the vaginal vault. The catheter is inserted until resistance is met. The resistance indicates that the cranial end of the vaginal vault has been reached or that the catheter has simply become trapped within vaginal folds. The catheter should be gently advanced as far cranially as possible before the semen is deposited to ensure deposition of spermatozoa near the cervix. Blind cannulation of the cervix is difficult because of its relative inaccessibility and anatomy (Roszel, 1992).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree