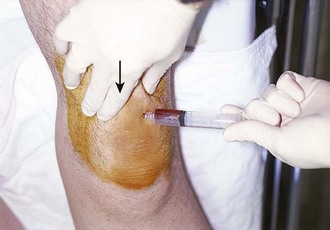
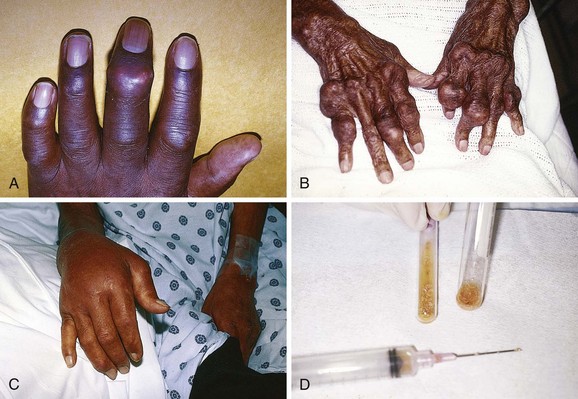
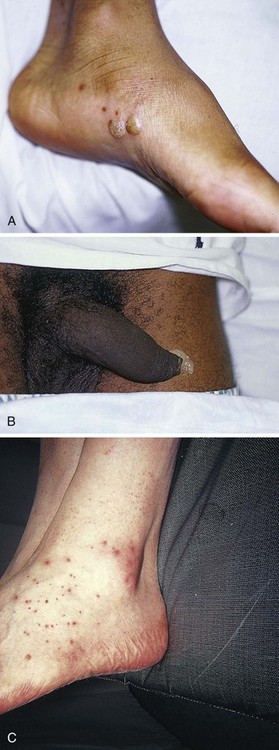
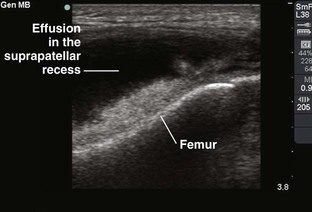
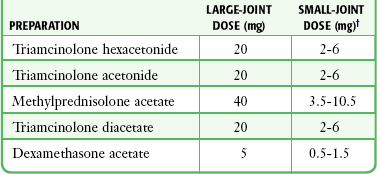
Chapter 53 Arthrocentesis, the puncture and aspiration of a joint, is an acknowledged, useful procedure that is easily performed in the emergency department (ED).1 It has been established as both a diagnostic and therapeutic tool for various clinical situations. When performed properly, the procedure offers a wealth of clinical information and is associated with few complications. In the ED it is difficult to make an accurate assessment of an acutely painful, hot, and swollen joint without performing arthrocentesis. The indications for arthrocentesis are listed in Review Box 53-1. Bleeding diatheses are rarely a relative contraindication, and arthrocentesis to relieve a tense hemarthrosis in bleeding disorders such as hemophilia is an accepted practice after infusion of the appropriate clotting factors. There are few data regarding the safety or dangers of arthrocentesis in patients taking anticoagulants or platelet inhibitors. Studies have demonstrated that the risk for iatrogenic hemarthrosis in patients treated with oral anticoagulants is extremely low, even in those who have international normalized ratios as high as 4.5.2 One prospective trial of 32 patients taking warfarin found no complications after athrocentesis.3 Hence, when necessary, arthrocentesis should be performed in patients taking anticoagulants. The value of reversing a coagulopathy with blood components before the procedure is not proved, and clinical judgment should prevail. Prosthetic joints are at high risk for infection, and arthrocentesis should be avoided whenever possible in this situation. However, if an infected prosthesis is suspected, arthrocentesis should be performed. If the swelling is secondary to joint effusion or inflammation, the entire articular capsule will be inflamed and distended and fluid can often be palpated within the joint. In the knee, this condition must be differentiated from effusion into the prepatellar bursa, where swelling distends the bursa that lies mainly over the lower portion of the patella, between it and the skin. Effusion into the joint occurs posterior to the patella, whereas bursal swelling occurs anterior to it (Fig. 53-1). When considerable articular effusion of the knee is present, the capsule of the joint is distended and an inverted U-shaped swelling of the joint develops. This characteristic shape occurs because the dense patellar ligament prevents distention of the capsule along its inferior border. Also, with the knee extended a large effusion causes the patella to “float” or lift away from the femoral condyles. Complete extension and flexion are often impossible because of the joint tension produced by the effusion. Acute monarticular arthritis is a common problem in emergency medicine. Although acute monarticular arthritis has many causes, septic arthritis is the one requiring most urgent diagnosis and treatment. Infectious arthritis is still relatively frequent, and suspicion of a septic process in the joint is the first step in appropriate management; confirmation requires arthrocentesis and culture of synovial fluid. In the ED, synovial fluid analysis is the diagnostic test most heavily relied on in making the diagnosis of an acute intraarticular infection. Culture remains the most definitive study, although it is not 100% sensitive.4 Gram stain may be helpful, but a negative Gram stain does not exclude the presence of a joint infection because not all infected joints have a positive Gram stain. Therapeutic arthrocentesis might be need to be repeated when treating a septic joint. Such therapy is usually performed on an inpatient basis.5 Infection of a joint occurs by one of several mechanisms: hematogenous spread (bacteremia, infective endocarditis, intravenous drug use), spread from a contiguous source of infection, direct implantation, postoperative contamination, or trauma.4 Septic arthritis is typically monarticular with a swollen, erythematous, and painful joint. The noninfectious differential diagnosis includes crystal-induced arthritis, fracture, hemarthrosis, foreign body, osteoarthritis, ischemic necrosis, and monarticular rheumatoid arthritis. In addition, osteomyelitis may mimic septic arthritis because of the close proximity of the infected metaphysis to the joint space.6 In many instances an acutely inflamed joint from gout or other arthritides simply cannot be distinguished from infection clinically. Nonetheless, early diagnosis is essential to prevent complications such as impairment of growth, articular destruction with ankylosis, osteomyelitis, and soft tissue extension.7 Because an acutely swollen joint may be indicative of a number of disease entities, a thorough history and physical examination are the cornerstones of evaluation, followed by arthrocentesis (Fig. 53-2). Laboratory findings can be useful in making a diagnosis, as can response to therapy (e.g., the response to empirical antibiotics in gonococcal arthritis is often the only criterion for diagnosis because the organism is difficult to culture). Blood cultures may be positive since joint infections may be due to hematogenous spread. Patients with malignancy (especially leukemia) or those who are immunosuppressed or otherwise debilitated are at particular risk for a septic cause. Infectious arthritis should be considered primarily in these patients, as well as in those with preexisting joint diseases such as rheumatoid arthritis. In general, a swollen joint is not usually injected with corticosteroids until the possibility of infection has been eliminated.8 Neisseria gonorrhoeae, Staphylococcus (including methicillin resistant), and Streptococcus are the most frequently identified etiologic agents. N. gonorrhoeae is the most common organism causing septic arthritis in adolescents and young adults. Patients older than 40 years and those with other medical illnesses are more likely to have Staphylococcus joint infections. In children, Staphylococcus, Streptococcus, and Escherichia coli predominate. Haemophilus influenzae was a common cause of pediatric septic arthritis in the past, but widespread use of the conjugate vaccine has reduced H. influenzae infection rates to nearly zero.8–10 In neonates, staphylococci, Enterobacteriaceae, group B streptococci, and N. gonorrhoeae are the most likely organisms. Staphylococcal or pseudomonal infections commonly develop in injection drug abusers. Salmonella arthritis is more prevalent in patients with sickle cell disease than in the general population; however, more common organisms still predominate. Prosthetic joints or postoperative infections have high rates of Staphylococcus aureus, Streptococcus epidermidis, Enterobacteriaceae, and Pseudomonas infection.11 The prevalence of community-acquired methicillin-resistant S. aureus (CA-MRSA) mandates special attention. Epidemiologic data on the incidence of CA-MRSA septic arthritis are sparse; however, one recent study noted that 50% of synovial fluid cultures in suspected septic arthritis ultimately grew MRSA.12 It would be prudent to consider empirical therapy for MRSA in those suspected of having septic arthritis until the results of culture become available. MRSA-infected joints can be multiple, progress rapidly, and be very destructive of joint tissue and adjacent bone. Although precise incidence data for nongonococcal septic arthritis have not been established, predisposing factors have been described and include age 80 years or older, diabetes mellitus, rheumatoid arthritis, hip or knee prosthesis, joint surgery, and skin infection.13 The simultaneous occurrence of gout and septic arthritis is possible, and one should not allow the establishment of a diagnosis of crystal-induced disease to stop a thorough search for infection.14 Because N. gonorrhoeae is the most common organism causing septic arthritis, gonococcal arthritis deserves special mention. Disseminated gonococcal infection occurs in 0.5% to 3% of cases of mucosal infection. Gonococcal septic arthritis is more common in women, especially during pregnancy or after menstruation, because women with sexually transmitted gonorrhea infections are more likely to be asymptomatic. The time needed for local infection to disseminate can vary from several days to weeks. Patients will often experience systemic symptoms, including fevers, chills, and malaise, as well as migratory polyarthralgia. Gonococcal tenosynovitis without joint involvement occurs in two thirds of patients. Dermatitis is also present in two thirds of patients (Fig. 53-3). The most common rash consists of scattered painless, nonpruritic 0.5- to 0.75-cm macules or papules with necrotic or pustular centers distributed on the extremities and trunk. Eventually, the infection settles into one or two large joints to yield a purulent arthritis.9,15 Overt urethritis and vaginitis may be absent or overlooked because of concentration solely on the obvious joint pathology. Hence, it is important to realize that disseminated gonococcal infections can be associated with surprisingly minimal or even absent signs and symptoms of a genital infection source. Some joints may become inoculated through hematogenous spread from anal and oral sites of infection. Even though N. gonorrhoeae–infected joint fluid is usually “septic” in character, the yield of positive synovial fluid cultures has ranged from 25% to 50%. Blood cultures appear to be less helpful since they are positive in only 20% to 30% of cases. Because blood and joint fluid culture has a low yield, it would be prudent to culture all possible sites of gonococcal infection, including anal and pharyngeal sites. The organism can often be identified in asymptomatic genitourinary sites,9 with cultures from the primary mucosal site being positive in up to 80% of infected patients.15 A positive Gram stain is immediately diagnostic of septic arthritis. However, Gram stains are positive in only 71% of gram-positive infections, 40% to 50% of gram-negative infections, and 25% of gonococcal infections.16,17 An elevated synovial white blood cell (WBC) count and a reduction in synovial fluid glucose may give confirmatory data. However, the synovial fluid WBC count in gonococcal arthritis is often between 10,000 and 20,000 cells/mm.3,4 Although mild leukocytosis and an elevated erythrocyte sedimentation rate may occur, normal laboratory values do not exclude infection.16,18 Isolated nontraumatic hemarthrosis may occasionally be seen by the emergency clinician. An inflammatory reaction may follow intracapsular bleeding, and the proliferative reaction and the hyperplastic synovium formed might predispose patients to recurrent hemorrhage in that joint, especially those with bleeding diatheses. The knee is the most commonly affected joint, followed by the ankle, elbow, shoulder, and hip.1 The most common cause of intraarticular hemorrhage in the setting of no or minor trauma is a hereditary clotting factor deficiency such as hemophilia. Hemarthrosis is an infrequent complication of oral anticoagulant therapy but might occur even with prothrombin times within the normal range.19 Cessation of anticoagulant therapy in these patients must be weighed against the risk for adverse clot formation (e.g., acute cerebrovascular accident). Chronic arthritis does not appear to be a long-term complication in patients with intraarticular bleeding from oral anticoagulant therapy. Hemarthrosis may also be a complication of sickle cell anemia, pseudogout, amyloidosis, pigmented villonodular synovitis, synovial hemangioma, rheumatoid arthritis, and infection.18,20 Distension of the joint by effusion or hemorrhage causes considerable pain and disability. If the fluid is not removed, it is partially absorbed, but part of it may undergo organization and result in the formation of adhesions or bands in the joint. This is one argument for drainage of the joint.2 Some believe that in an otherwise healthy joint that is subjected to a single traumatic event, even a relatively large hemarthrosis will be spontaneously reabsorbed without significant sequelae and therefore presents no pressing need for drainage. Unfortunately, no literature exists to guide the best approach; hence, there is no universal standard of care regarding the need or lack thereof of draining blood from a traumatic joint. Nonetheless, a large, tense, traumatic effusion is quite painful, and its presence precludes proper evaluation of an injured joint. Therapeutic arthrocentesis to drain a symptomatic traumatic effusion is a common and well-accepted practice.2,19,21,22 The source of blood after trauma is frequently a tear in a ligamentous structure, capsule, or synovium or a fracture. Cruciate (especially anterior) ligament injury is the most common cause of significant hemarthrosis after trauma to the knee.20 A joint effusion that develops 1 to 5 days after trauma may be secondary to a slow hemorrhage or reinjury, but the swelling is often caused by a nonhemorrhagic irritative synovial effusion. Occasionally, one will diagnose an occult fracture by the presence of lipohemarthrosis, or fat globules in the arthrocentesis specimen (Fig. 53-4). This may be appreciated when the bloody effusion is placed in a clear container (e.g., emesis basin) and held to a light. If the history of trauma is vague, arthrocentesis may be required to differentiate hemorrhage from other causes of joint effusion. An occult tibial plateau fracture is an example in which evaluating for lipohemarthrosis may be of particular value. Following therapeutic arthrocentesis for a hemarthrosis, it may be desirable to inject 2 to 15 mL, depending on joint size, of a long-acting local anesthetic (see Chapter 29) into the joint to facilitate examination or provide temporary relief of the symptoms. In 1951 Hollander and coworkers23 first demonstrated that intraarticular corticosteroid injections are useful for relief of symptoms in patients with severe rheumatoid arthritis. The use of steroids has proved to be a dependable method for providing rapid relief of pain and swelling of inflamed joints, although it is strictly local, usually temporary, and rarely curative.2,24,25 It is easily performed in the emergency setting. Acute gout responds well to joint injection, and this may be preferable in patients who cannot tolerate indomethacin or colchicine. Corticosteroid injections are most helpful when only a small number of joints are actively inflamed. The most frequently used corticosteroids for intraarticular injection are shown in Table 53-1.2 Diminution of joint pain, swelling, effusion, and warmth is usually evident within 6 to 12 hours after injection. TABLE 53-1 Intrasynovial Corticosteroid Preparations* *Listed in approximate descending order of duration of action. †The dose will depend on joint size, capsular distensibility, and degree of inflammation. From Gray RG, Gottlieb NL. Corticosteroid injections in RA: appraisal of a neglected therapy. J Musculoskelet Med. 1990;7:53. Reproduced by permission. Though very rare, the most serious complication of this practice is intraarticular infection.2 Therefore, steroids should not be injected into a joint if a joint space infection is suspected. Repeated injections into one joint pose a risk for necrosis of juxtaarticular bone with subsequent joint destruction and instability and suppression of the hypothalamic-pituitary axis from systemic absorption. Other complications include local soft tissue atrophy and calcification, tendon rupture, intraarticular bleeding, and transient nerve palsy.2,25 Transient elevations in blood glucose, as well as erythema, warmth, and diaphoresis of the face and torso, may also occur after intraarticular steroid injections. Acute pain, redness, and swelling 12 to 24 hours after steroid injection can mimic infection, but with this timing it is most likely an inflammatory reaction (steroid flare) to crystal-containing steroid preparation (often methylprednisolone). Deposition of steroid crystals on the synovium might give rise to a transient, self-limited flare-up of synovitis.2,26 It is always important to determine whether local corticosteroid therapy has been used previously, not only to consider the array of clinical conditions associated with steroid use but also because crystalline corticosteroid material can hinder proper interpretation of crystals found in synovial fluid.26 The material needed for arthrocentesis includes skin preparation solutions (e.g., chlorhexidine); sterile gloves and drapes (optional in some cases); local anesthetic; syringes for injecting local anesthetic and aspirating joint fluid; a three-way stopcock for draining large amounts of fluid; lavender-, red-, and green-topped blood tubes; and various sizes of needles and intravenous catheters (see Review Box 53-1). Depending on the size of the effusion to be drained, a 10-, 20-, or 30-mL Luer-Lok syringe can be used. If a large effusion is suspected, a three-way stopcock between the needle and the syringe allows complete drainage with a single joint penetration. Fluid for cell count should be collected in a lavender-topped tube; however, viscosity, protein, and glucose determinations do not require anticoagulants, and fluid should be placed in a red-topped tube. Though still common practice in many institutions, recent evidence suggests that synovial fluid protein and glucose levels are poor differentiators of noninflammatory and inflammatory effusions and are no longer recommended (see “Synovial Fluid Interpretation,” later).2,27–29 Immediately examine fresh synovial fluid in its unadulterated form for crystals. Calcium oxalate and lithium heparin anticoagulants have been reported to introduce artifactual crystals into the fluid. Joint fluid to be analyzed for crystals should be collected in a green-topped tube containing sodium heparin. If culturing for N. gonorrhoeae, the fluid should be immediately placed on proper medium and stored in a low-oxygen environment in the ED.
Arthrocentesis
Background
Indications and Contraindications
Articular versus Periarticular Disease
Septic Arthritis
Hemarthrosis
Intraarticular Corticosteroid Injections
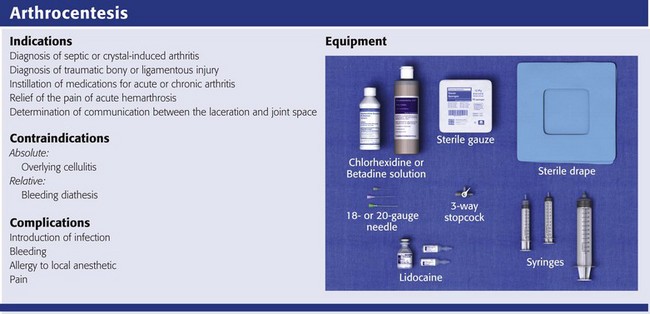
Equipment
Arthrocentesis