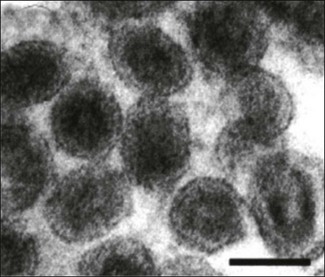
Chapter 58 Arteriviruses were formerly classified as members of Togaviridae on the basis of morphological similarities. However, their genome organization and replication strategy is similar to members of Coronaviridae. A new family Arteriviridae was created by the International Committee on Taxonomy of Viruses in 1996. It is part of the Order Nidovirales, which also includes the family Coronaviridae. The name of the family is derived from equine arteritis which is the disease caused by the type virus species. Arteriviruses are medium-sized, spherical viruses, 50–74 nm in diameter (Fig. 58.1). They have an envelope which possesses a surface pattern of small, indistinct projections. The isometric nucleocapsid contains a single molecule of linear, positive-sense, single-stranded RNA. As well as the nucleocapsid protein (N) there are two major (GP5, M) and four minor (GP2, GP3, GP4, E) envelope proteins. Twelve non-structural proteins (nsp1–12) have been described. Replication occurs in the cytoplasm of infected cells. Nucleocapsids bud into the lumen of smooth endoplasmic reticulum and are released from infected cells by exocytosis. Arteriviruses are quite labile and are sensitive to heat, UV irradiation, low pH, lipid solvents, detergents and many disinfectants. Figure 58.1 Electron micrograph of negatively stained particles of porcine reproductive and respiratory syndrome virus particles. Bar is 50 nm. Reprinted with permission: Fauquet, C.M., et al. (Ed.), 2005. Virus Taxonomy Eighth Report of the International Committee on Taxonomy of Viruses. Elsevier Academic Press, pp. 965. There is a single genus Arterivirus (Fig. 58.2). Members of the genus infect horses, pigs, mice and monkeys (Table 58.1). They are not antigenically related and are host-specific. Spread of infection is horizontal and includes aerosol, biting and venereal transmission. The primary target cells are macrophages and persistent infections are frequently established. Table 58.1 Overview of disease significance of arteriviruses The percentage of seropositive horses varies according to breed and country. In general seroprevalence is high among standardbreds but low among thoroughbreds. It is unclear whether this difference reflects differences in susceptibility or differences in risk of exposure due to management practice differences. Equine arteritis virus is spread during the acute phase of infection primarily by aerosols from respiratory secretions. Close contact is required for the efficient spread of the infection. Mares and geldings eliminate the virus within one to two months of infection but about 35% of infected stallions become persistently infected. Carrier stallions are asymptomatic but shed the virus continuously in their semen and can venereally transmit the infection to 85–100% of mares covered by them. Persistent infection does not impair the fertility of stallions and appears to be testosterone-dependent (McCollum et al. 1994). Mares infected venereally may return to the home farm and spread the infection horizontally to in-contact susceptible animals. Infected pregnant mares can transmit the virus transplacentally to their foetus resulting in abortion or the birth of an infected foal. Equine arteritis clinically resembles a number of other infectious diseases and definitive diagnosis requires laboratory confirmation. Internationally accepted testing procedures have been published (Timoney 2008). • Virus isolation can be carried out in permissive cell lines such as rabbit or equine kidney. Early passage RK-13 cells are the cell system of choice. Appropriate samples include nasopharyngeal swabs, conjunctival swabs or placenta, foetal tissues and fluids. Heparinized blood is not suitable because of the inhibitory effects of heparin. Visible CPE is usually evident within two to six days. • The characteristic vascular lesions found in affected adults are not a feature of EVA-related abortions but viral antigens can be detected in placental and foetal tissues by immunohistochemistry. • Viral RNA can be detected in semen and other specimens using reverse transcription-polymerase chain reaction. Single, nested and real-time RT-PCR protocols have been described (Belak et al. 1995, Gilbert et al. 1997b, Belasuriya et al. 2002). A universal primer set has not been agreed upon yet and it is recommended that RT-PCR should be used in conjunction with virus isolation (Timoney 2008). • Acute and convalescent blood samples should be collected for serological testing. Several serological tests including virus neutralization, complement fixation, indirect fluorescent antibody, agar gel immunodiffusion and ELISA are suitable. The virus neutralization test (Senne et al. 1985) is considered to be sensitive and highly specific. It is the most widely adopted test and is typically carried out in microtitre plates with the addition of complement to enhance sensitivity. The first ELISAs developed tended to identify a high number of false-positives due to the presence of antibodies to tissue culture antigens in horses vaccinated with tissue culture-derived vaccines (Cook et al. 1989). Improved performance has been achieved by the use of more specific reagents such as recombinant major envelope GP5 (previously known as GL) protein as antigen (Hedges et al. 1998, Nugent et al. 2000) or monoclonal antibody to the major envelope GP5 protein in a blocking ELISA (Cho et al. 2000). • In the case of carrier stallions, semen should be collected for virus isolation from animals with positive titres. The sperm-rich fraction of a semen ejaculate is suitable for virus isolation or RT-PCR. An alternative strategy is to mate such animals to seronegative mares that are then monitored for seroconversion.
Arteriviridae
Virus
Host species
Disease
Equine arteritis virus
Horse
Rhinitis, conjunctivitis and oedema of the legs and trunk. Abortion is common in pregnant mares
Porcine reproductive and respiratory syndrome virus
Pig
Reproductive failure, pneumonia in piglets and increased pre-weaning mortality
Lactate dehydrogenase-elevating virus
Mouse
Life-long persistent viraemia with no clinical disease usually. Elevated enzyme levels in blood
Simian haemorrhagic fever virus
Macaque monkey
Haemorrhagic fever in macaques, other monkey species may carry the virus
Equine viral arteritis (EVA)
Pathogenesis
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Arteriviridae
Only gold members can continue reading. Log In or Register to continue