, Monika Hassel2 and Maura Grealy3
(1)
Centre of Organismal Studies, University of Heidelberg, Heidelberg, Germany
(2)
Spezielle Zoologie, Universität Marburg FB Biologie, Marburg, Germany
(3)
Pharmacology and Therapeutics, National University of Ireland Galway, Galway, Ireland
In this chapter experiments are introduced that originally were designed to answer scientific questions but subsequently turned out to be useful in stockbreeding, medicine, pharmaceutics, or biotechnology. Basic research leading, for instance, to artificial teratomas, is often not of benefit for practical medicine. Nevertheless by these experiments knowledge was gained about how malformation can develop also in humans. Some of the subsequently described and discussed experiments, in particular cloning of mammals, caused some anxiety and controversies, and therefore hit the headlines of the media.
13.1 Cloning, the Production of Identical Copies
13.1.1 Cloning Is a Natural Event in the Plant World and in Many Non-vertebrates, and Plant Breeding Has Supplemented the Natural by Artificial Procedures from Time Immemorial
In present day biology the term cloning is most frequently used in connection of work in molecular biology, and refers to the, hopefully faithful, multiplication (amplification) of DNA sequences in bacterial hosts. Molecular biologists have adopted the term from classical biology. In traditional biology a cellular or organismal clone is a collection of genetically identical cells or multicellular organisms; cloning is the production of such clones. Natural cloning, traditionally called vegetative reproduction, is known from many plants and invertebrates, and is based on mitotic proliferation. Reproduction through vegetatively produced tubers (for example potato), through offshoots or stolons (for example strawberries, hydrozoan colonies, coral polyps), through buds (for example the plant Bryophyllum or the fresh water polyp Hydra) are forms of natural cloning.
In plant breeding the natural procedures are supplemented by artificial methods of reproduction. Since times immemorial currants are propagated by cutting off twigs and inserting them into humid ground where the grow roots (cloning by regeneration). Fruit-bearing trees and vine stocks are propagated by grafting, that is by transplanting twigs under the bark of a rooted tree. In this way cultivated varieties have been bred which all look alike, have the same nutrient content, and taste alike, and are distributed all over the world.
The above examples show the benefits the farmer, vintner, and gardener can expect from cloning. He/she can be sure that all descendants have the same features as has the stem organism. Not so in sexual reproduction via seeds. As in sexual reproduction of humans it is indeterminate how offspring will turn out.
In current plant breeding many plants which cannot be multiplied by natural vegetative reproduction are propagated via protoplasts, these are cells taken of existing plants deprived of their cell wall and grown in culture where they form multicellular clumps, called callus. These can be induced to form vegetative embryos, without intermediary sexual processes.
In animal breeding vegetative reproduction has not yet yielded procedures that can be used in biotechnology. There is no commercial interest in cloning sponges, hydrozoans, corals or ascidians which would be easily possible. In vertebrates (except in polyembryonic armadillos) naturally cloned animals are the result of an accident when blastomeres of early embryos fall apart and monozygotic twins are born (Fig. 13.1). Yet, the breeder would be happy if he/she could clone a successful racehorse (it is possible but extremely expensive). Currently there is much interest in cloning transgenic animals that were generated with arduous work for medical research. This will be discussed in Sect. 13.3.4.
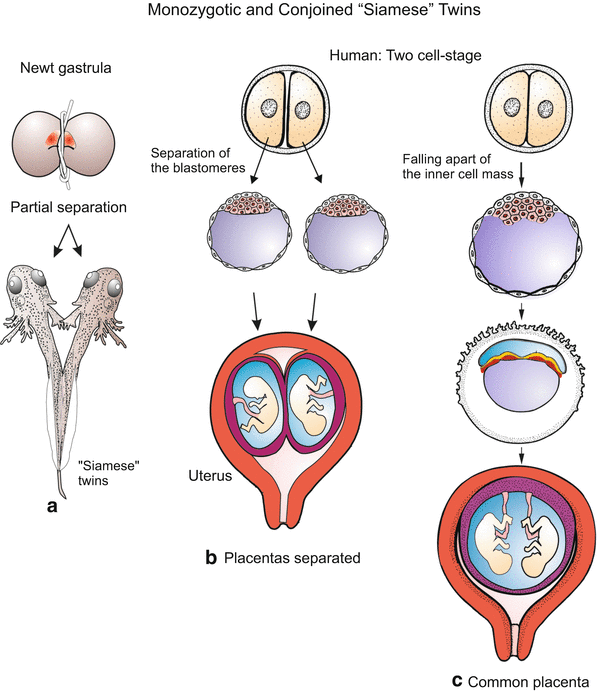

Fig. 13.1
Monozygotic twins in amphibians and humans. Upon partial separation of early amphibian embryos partially connected “Siamese” twins arise whereas complete separation gives origin to two complete tadpoles. In humans the first two blastomeres can fall apart, or the inner cell mass breaks into two parts. In the first case two monozygotic twins grow in separate envelopes, in the second case the twins grow in a common envelope (chorion)
13.1.2 A Biotechnologically Irrelevant Cloning Procedure Is Dissection of Early Embryos
Mini-clones consisting of identical monozygotic twins (actually two, four or eight individuals) arise when the first two, four or eight blastomeres fall apart or are separated experimentally. In mammals, each separated blastomere may give rise to a small but complete blastocyst which implants and develops into a fetus with its own placenta. Since mammalian embryos are supplied with nutrients multiples can grow to normal size provided space in the womb is large enough. In mammals, including humans, even the inner cell mass of the blastocyst may fall apart into two or more groups: those twins are found within a common chorion (embryonic envelope, Fig. 13.1c) and have a common placenta.
Partial division of an early embryo results in conjoined twins, in common parlance called “Siamese” twins. The emergence of such twins was elucidated by H. Spemann by partially constricting early embryos of newts (Fig. 13.1a).
13.1.3 Cloning by Transplantation of Somatic Nuclei: Pioneering Experiments in Xenopus Opened Means to Breed Many Progeny with Known Characteristics
Clones comprising many animals were first produced in the clawed toad Xenopus (by R.W. Briggs, T.J. King, J.B. Gurdon, Fig. 13.2). From activated egg cells the zygotic nucleus is removed with a micropipette or destroyed by UV irradiation. A substitute nucleus is removed from a suitable somatic cell type taken from a donor animal and transferred into the enucleated egg by means of a glass micropipette. Fortunately, in amphibians pricking with a micropipette suffices to activate the egg.
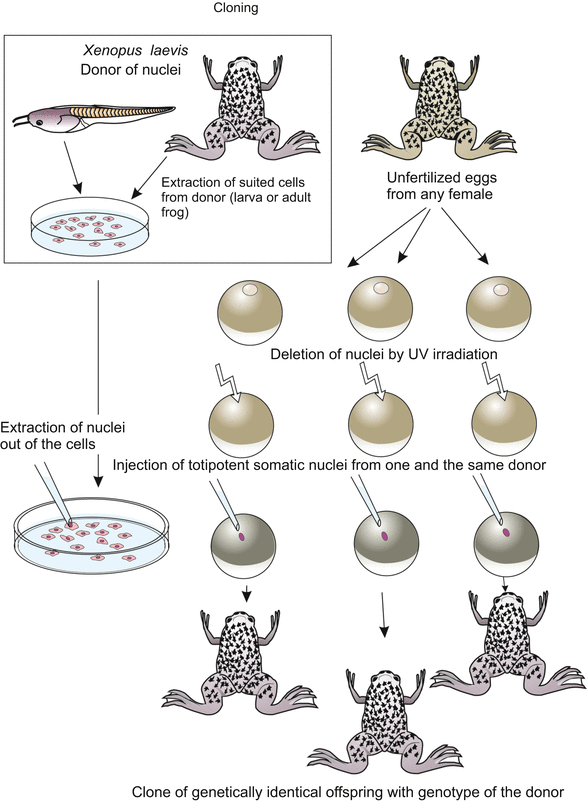

Fig. 13.2
Cloning of Xenopus. Totipotent nuclei taken from somatic cells of a donor are injected into oocytes the nuclei of which were previously destroyed by a UV microbeam. Suitable donor nuclei can be extracted from tadpole cells (e.g. from cells of the intestine) or even from adults (e.g. lung cells)
Originally the experiment was designed to check whether somatic cells retain totipotency. Suitable totipotent donor cells were first found in the intestine of tadpoles and later in several tissues of adult animals. To produce numerous clones the procedure is repeated many times using the same donor animal: all offspring will be genetically identical with the donor and with each other.
13.1.4 Cloning of Mammals Meets Particular Problems; So Foster Mothers Must Cooperate
Unlike frog eggs oocytes of mammals must be surgically removed from the maternal oviduct or the ovary. Besides, the oocytes must be activated in some way, for example by fertilization with a sperm and subsequent removal of the male pronucleus, or by a well-dosed electric pulse. And mammalian eggs do not develop in water outside a mother. They must be kept in particularly prepared media up to the blastocyst stage and then transferred into the uterus of nurse females prepared to accept the egg by injection of gonadotropic hormones. The animal from which the host egg originally was removed is, as a rule, too much affected to accept and bear the child. A surrogate mother, also known as foster mother (Fig. 13.3) must step in.
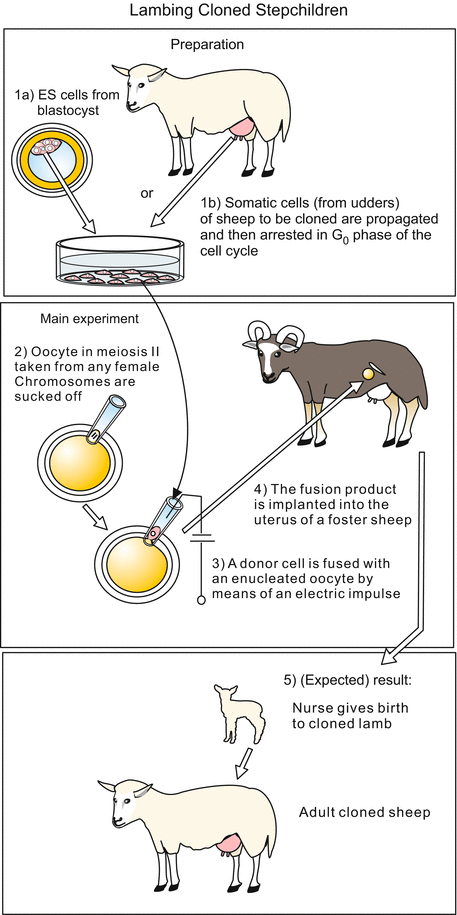

Fig. 13.3
Cloning of sheep. Large enucleated oocytes are fused with small somatic donor cells taken from a suitable tissue. Donor cells can be removed from a blastocyst or taken from a tissue of adult animals that contains proliferating cells, for example from milk glands. Before fusion the cells are propagated in culture and eventually locked in the G0 phase (phase of quiescence) of the cell cycle. Fusion is induced by an electric impulse. The fusion product starts cleavage in a suitable medium. The blastocyst stage is transferred to the uterus of a ewe, where it develops to term
13.1.5 Cloning of Mammals Using Embryonic Donor Nuclei Is Possible for Quite Some Time; Though the Dream Goal Has Not Yet Been Achieved
Using the technique developed in Xenopus cloning of mammals was initially only successful if the nuclei were taken from
Blastomeres of two-cell through eight-cell stages, before compaction, or from
Embryonic stem cells (ES-cells, ESC) that is cells removed from the inner cell mass of a blastocyst. Previous to the transfer experiment the totipotent ES cells are multiplied through several passages in culture so that many genetically identical donor cells are available and many egg cells can be provided with a foreign nucleus.
In a next step the difficult technique of transferring a nucleus was replaced by a simplified technique. First the nucleus of the host oocyte is removed by sucking it out with a fine glass pipette. The mammalian oocyte is surrounded by a jelly-like coat known as zone pellucida, and within this coat is inserted one cell from a donor, a blastomere or an ES-cell. Fusion of the oocyte and the donor cell is induced by administering an electric pulse. Through this electrofusion a nucleus is reintroduced into the oocyte forming a reconstituted egg. (However, the donor cell can bring along disturbing cytoplasmic components such as transcription factors, as discussed below in Sect. 13.1.6.)
After nuclear transplantation has been accomplished, the host eggs are transferred to nurse females prepared to accept the egg by injection of gonadotropic hormones. Such nurses or foster mothers have given birth to lambs, cattle, pigs and rabbits. For a long time cloning was not successful if nuclei from differentiated cells were injected. In mice, even nuclei from four-cell stage embryos were unable to support full development.
Cloning of animals using cells from embryos or fetuses is not what is desired in breeding of farm animals because the future phenotypic outcome of a genome taken from an embryonic donor genome is unknown. Therefore, the technique as described was only a preparatory step towards the main goal, cloning of adults with known characteristics.
13.1.6 Cloning with Nuclei Taken from Adults Is Possible with Restrictions: Dolly Was the First Proof
The aspiration of breeders was to clone animals (race horses for example) whose performance was known and proven. Therefore donor nuclei from adults are in demand. After many years of experimenting in 1996 eventually a first Scottish domestic sheep was cloned, widely known as Dolly. Instrumental for the success were several proceedings:
Oocytes in the stage of final maturation (1st polar body just extruded) were selected as host cells, and proliferating cells were selected as the donors of nuclei, actually putative unipotent stem cells from the mammary milk glands which could be cultivated and multiplied before use (the meaning of unipotent is explained in Chap. 18 and in the Glossary).
The donor nuclei were brought into a suitable state. Before use the donor cells were deprived of serum to bring them into the quiescent G0 phase of the cell cycle. Egg cells divide rapidly, somatic cells slowly, even when kept in culture. It proved successful in practise to arrest the donor cell in the G0-phase of the cell cycle. In this phase the time-consuming transcription of genes comes to a standstill. Apparently from this quiescent state a quicker restart with rapid succession of nuclear replication is possible.
With a well-dosed electric pulse not only fusion of the egg cell with the donor cell but also activation of the egg cell was achieved.
In culture medium containing components of the uterine secretion the manipulated eggs developed up to the blastocyst stage capable of implanting itself into the uterus of a surrogate mother.
The production of Dolly showed that genes in the nucleus of a somatic partially-committed stem cell is still capable of reverting to an embryonic totipotent state, apparently rendered possible by factors present in the cytoplasm of egg cells. We will later in Chap. 18 see that even terminally differentiated cells can provide totipotent nuclei if certain pluripotency genes are reactivated.
In 1998 the first cloning of mice succeeded when potentially totipotent nuclei were found in cumulus cells. These are relicts of maternal tissue attached to the surface of freshly ovulated eggs. (In numerous previous experiments in vitro blastocysts, once implanted into a foster mother, died before term because the trophoblast did not develop a normal placenta.)
Cloning succeeded, for example, in domestic sheep (Dolly 1996), in the house mouse (1998), domestic cattle (1998), domestic goat (1998), domestic pig (2000), mouflon (2000), gaur (Indian bison) (2001), domestic rabbit (2001), domestic cat (2001), brown rat (Rattus norvegicus, 2002), in the mule (2003) and domestic horse (2003), in the African wild cat (2003), in the red deer (2004), ferret (2004), Asian water buffalo (2005), the Afghan hound dog named Snuppy (2005) and the grey wolf (2005). In 2007 rhesus monkeys were cloned for the first time, and in 2009 an Arabian camel. Currently (2012) race horses with great sporting prowess are cloned in notable numbers. In 2008 in South Korea seven cloned drug-sniffing dogs (Golden Retriever) were born who, such the hope of the customs authorities, should be as successful as was the original. The aim is also to clone endangered species such as marsupials but there is lack of donors of oocytes and of surrogate mothers.
13.1.7 There Have Been Many Disappointments Because of Hampering Epigenetics
The fact that the success rate of cloning mammals was (and remains) very low, between 0 and 4 % at the maximum – was and is unfortunate for the client and reproductive veterinarian as well. The frustrating experience, and not caused by mere technical deficiency, was that many fetuses were lost by abortion or died shortly before term. Moreover, the observation that not all offspring were exactly identical copies of the donor of the nucleus appeared to contradict classical theories. Dolly aged too early (presumably partly caused by infectious diseases). Too high birth weight, premature ageing, and physical deficiency have been diagnosed. Several causes are discussed:
Incomplete reconstitution of the chromosomes back to the juvenile state. Chromosomes experience secondary, epigenetic modifications during differentiation such as methylation and acetylation of the DNA and the DNA-associated histones (see Figs. 12.22 and 12.23), or heterochromatinization of large parts of chromosomes. These epigenetic modifications reduce the accessibility of genes for transcription and are the cause of phenomena known as “genomic imprinting”, “epigenetic memory” and “gene silencing” (Sect. 12.5). In the oocyte advantageous conditions support the reconstitution of the juvenile state; after all the highly methylated and condensed chromatin of the sperm nucleus must be brought into a state allowing transcription. For instance, the cytoplasm of the egg cell contains many demethylases. However, the resetting of the status of chromatin is often incomplete, even in the chromosomes supplied by the oocyte and the sperm. Hence maternal or paternal imprinting can be observed (Chap. 8). In cloning by means of somatic nuclei incomplete resetting of the chromatin may hinder accessibility of important genes. In addition in the nuclei of differentiated cells many transcription factors not yet present in the egg cell are bound to DNA.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


