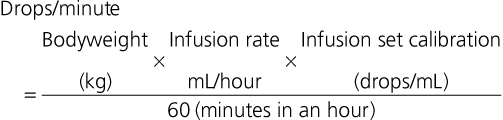Chapter 10
Apparatus for administration of anaesthetics
Anaesthetic induction chambers
Filters and humidity and moisture exchangers
Troubleshooting the delivery circuits
Administration of intravenous agents
Agents that are intended to reach the central nervous system (CNS) and produce narcosis or anaesthesia have the most direct route by intravenous (IV) injection.
However, it must be remembered that IV administration results in a more rapid onset and intense effect of drug action than achieved by other routes. Precise control of drug administration is essential and this can be influenced by the size of needle or syringe chosen and the use of syringe pumps and fluid infusion pumps.
Any superficial vein may be used for IV injection and detailed descriptions of the techniques of venepuncture are given in chapters describing anaesthesia in the various species of animal.
Syringes, needles, and catheters
Syringes used in veterinary medicine vary in size from 0.5 mL (insulin syringes) to 60 mL capacity. The nozzles of the syringes may be centred, or eccentrically placed to facilitate percutaneous venepuncture. Hypodermic disposable needles are sharp but the bevels on catheter needles may be shorter to aid placement within the vein. Desensitization of the skin in small animals prior to venepuncture may be achieved by application of a cream containing lidocaine and prilocaine to the skin and covering the area with an occlusive bandage for 30 minutes. In large animals, desensitization is achieved by injection of an intradermal or subcutaneous bleb of 2% lidocaine with a 25 gauge needle.
Administration of supplemental doses of anaesthetic agents to prolong anaesthesia or administration of fluids and antibiotics during anaesthesia requires a venous access. The simplest version is to place a needle in the vein and leave it with the loaded syringe taped to the patient. Not uncommonly the needle is displaced from the vein when the animal is moved, resulting in haematoma formation, injection of drugs perivascularly, and failure of ability to inject anaesthetic agents when needed. Improved security is achieved using a needle with wings on the hub and a variable length of extension tubing (Fig. 10.1). These needles are usually restricted to short anaesthetic procedures or specific needs.
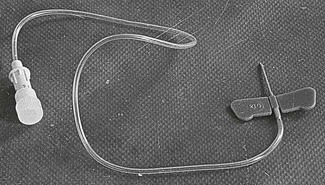
Figure 10.1 ‘Butterfly’ or ‘small vein set’ or ‘infant scalp vein’ set. The winged needles aid insertion and subsequent fixation to the patient. The attached plastic tubing allows injection without disturbing the needle.
A safer practice is to place a catheter in a vein before induction of anaesthesia (Fig. 10.2). The catheters available are purchased in sterile packs and are available in a variety of materials, such as polyvinylchloride, polypropylene, polyethylene, polytetrafluoroethylene (Teflon), and polymerized silicone (silastic). Catheters constructed of polyvinylchloride, polyethylene, and polypropylene are flexible, catheters of silastic or polyurethane have extreme flexibility, and Teflon catheters have minimal flexibility. The catheter materials exhibit varying thrombogenicity, with silastic having none. Many patterns of ‘over the needle’ catheters are available. There is some variation on their general shape and some have small handles to aid insertion. Most have plastic needle hubs (flash chamber) or caps through which blood can be seen when the needle enters the vein. Long catheters are chosen for placement in the jugular or saphenous veins.
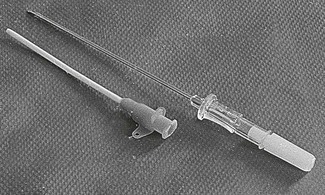
Figure 10.2 Disposable ‘catheter over the needle’. The point of the hollow metal needle projects beyond the tapered end of the catheter.
The choice of catheter size depends on the size of the animal and the purpose for which it is intended. The flow of liquid through a tube is proportional to the driving pressure, which is equal to the pressure difference between the two ends of the tube, and to the internal diameter of the tube, flow being directly proportional to the fourth power of the radius, and inversely proportional to the length of the bore. Flow is also inversely proportional to the viscosity of the fluid, since the more viscous it is the harder it will be to force it through the tube. Thus, for maximum flow of any given liquid at any given pressure, the tube should be short and the diameter large. Furthermore, a small change in diameter has a great effect on flow velocity.
At very high flow rates, the resistance to flow may be disproportionately high. There is a critical flow velocity at which flow changes from linear to turbulent. During turbulence, the driving pressure is largely used up in creating the kinetic energy of the turbulent eddies. The flow no longer depends on the viscosity of the fluid but on its density. The critical velocity at which turbulence occurs depends on the viscosity and density of the fluid and the radius of the tube through which it is flowing. Turbulence will also occur at points in the infusion apparatus where the internal diameter abruptly changes.
The viscosity of blood is greater than that of water, and increases with increased haematocrit (packed cell volume). Viscosity is also increased at low temperatures and the viscosity of blood at 4°C is about 2.5 times as great as at 37°C. Warming blood prior to transfusion will increase flow rate as well as decrease the impact on body temperature.
Skin preparation before insertion of the catheter, securing the catheter, and attentive catheter maintenance are essential to avoid catheter-related blood stream infection. Chlorhexidine is recommended as the best skin antiseptic for providing protection against catheter colonization (Norwood & McAuley, 2005). The catheter should be securely attached to the skin because movement of the catheter in and out of the site of skin insertion increases bacterial contamination. When catheters are to remain in place for an extended time, they should be protected from the environment by sticky pads or bandaging. The catheter site should be checked at least daily for signs of redness, swelling, or discomfort for the animal. Wet or soiled bandages should be changed whenever contamination occurs. Recommendations for changing catheters vary and while some recommend every 72 hours to decrease the risk of phlebitis and catheter-related infections, others suggest a longer retention time is acceptable if the site of catheter insertion appears healthy and daily maintenance is strictly observed. Catheter-associated infection rates of 15–49% have been reported in dogs and cats. A positive culture rate of 24.5% out of 101 central and 50 peripheral catheters in dogs and cats staying a minimum of 48 hours in an ICU unit was recently reported (Marsh-Ng et al., 2007). Since this was a prospective study, careful attention to aseptic technique and bandaging was observed. The three most common bacteria isolated from the catheter tips were not normal skin flora and it was deduced that transmission of bacteria from human hands to the catheter hub or contamination of the catheter cap were most likely causes. There were no differences in contamination rates between the types and location of catheters. Another investigation identified contamination in 23 of 99 (23.2%) of peripheral catheters removed from dogs and cats, most commonly Staphylococcus aureus and S. intermedius (Jones et al., 2009). None of the usual variables associated with catheter contamination, such as number of attempts at insertion and duration of placement, was associated with contamination and, although the statistical analysis was not conclusive, it appeared that catheters fitted with a Y-connector were 10 times less likely to be contaminated than catheters with a T-connector. The authors postulated that the reason may be that there is a greater distance from the injection port and the catheter when using a Y-connector.
Techniques of catheter insertion differ slightly between species, and are described in the species-specific chapters, but basic principles are described in Box 10.1. In some patients, insertion of the needle and catheter through the skin results in crimping, fraying or expansion of the end of the catheter and, in these cases, a small incision in the skin before insertion may be necessary. When venepuncture is unsuccessful, even when the needle has been only partially withdrawn from the catheter, the needle should not be reinserted into the catheter when still in the patient. If the catheter has been bent, reinsertion of the needle will result in the needle penetrating the side of the catheter and may even shear off the end of the catheter. Safe practice involves removal of the needle and catheter and starting again.
Catheters inserted into jugular veins of dogs, cats, and small farm animals are frequently placed using a modified Seldinger technique that requires inserting a guide wire into the vein to facilitate subsequent insertion of the catheter. Hair is clipped from an area over the jugular vein and the skin is prepared as for surgery. Sterile gloves are worn and a sterile drape with a hole in the centre is applied to isolate the site for catheter insertion. Jugular catheters may have one, two or three lumina for infusion of fluids, drugs and blood sampling (Fig. 10.3). Before inserting the catheter, the proximal and medial lumina should be flushed with heparinized saline and capped to prevent air entrainment. A catheter, or a needle, is inserted into the jugular vein pointing towards the heart. A spring guide wire, with or without a flexible J-tip, is threaded through the catheter into the jugular vein and then the catheter is removed while the guide wire is held stationary. A scalpel blade can be used minimally to enlarge the skin incision around the guide wire. A tapered tissue dilator is threaded over the guide wire into the vein using a partial back and forth rotation about its longitudinal axis to enlarge the path through subcutaneous tissue, and then removed. The catheter then is introduced into the jugular vein over the guide wire, which is then removed. The catheter is secured with sutures to the skin. The Seldinger technique using a guide wire can also be used to insert catheters into arteries, and the catheter guide wire can be purchased individually or is packaged as an integral component of the catheter.
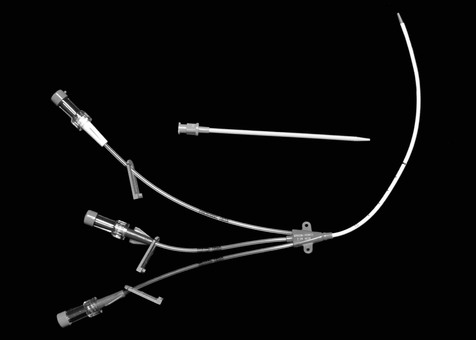
Figure 10.3 A triple lumen catheter that can be inserted percutaneously into a vein using the modified Seldinger technique involving a short catheter, a flexible guide wire (not shown), and a tapered tissue dilator.
Intraosseous needles
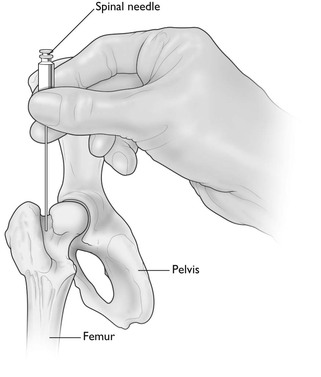
An intraosseous (IO) needle is used in animals in which venous catheter placement is particularly difficult, such as very small puppies and kittens and birds, and for emergency resuscitation. This route can be used for administration of crystalloid and colloid solutions, blood, and resuscitation drugs. Speed of infusion is limited. However, administration of fluids may expand the peripheral circulation sufficiently to facilitate subsequent intravenous catheterization. An IO needle should not be inserted through infected skin or into a fractured bone or pneumatic bone in birds. Aseptic technique must be employed to prevent introduction of bacteria and osteomyelitis. In dogs and cats, the sites of needle insertion most commonly used are the trochanteric fossa of the femur, the greater tubercle of the humerus, and the medial surface of the proximal end of the tibia (Fig. 10.4). In birds, the needle is inserted into the proximal end of the ulna. Local infiltration of local anaesthetic solution or general anaesthesia should be administered. Purpose-made IO needles are commercially available. Other options are 22, 20, or 18 gauge spinal needles, hypodermic needles for extremely small patients, and bone marrow needles for large dogs. A stab skin incision with a scalpel blade may be needed to assist insertion of the needle. The needle should be introduced with pressure using a partial back and forth rotation about its longitudinal axis achieved by rolling the needle hub between thumb and forefinger. Loss of resistance may indicate penetration of the cortex. The stilette of the needle is removed and a 3 mL syringe attached. Confirmation of placement is by aspiration of bone marrow into the syringe and then easy injection of heparinized saline with no swelling occurring subcutaneously. A catheter cap or T-port should be attached to the needle. Antibiotic ointment can be smeared at the site of skin insertion and the needle should be secured with tape or sutures. The flow rate of fluid through the needle is not significantly altered by using a large needle size but is dependent on flow through the bone marrow. Pressurizing the source of the fluid doubles the rate of fluid infusion but should be used cautiously. More details about IO infusion have been previously published (Giunti & Otto, 2009).
Vascular access ports
Vascular access ports may be used in patients that need long-term daily intravenous medications, frequent blood sampling, or multiple anaesthetic episodes for radiation therapy. A soft catheter is inserted into a vein, usually the jugular vein, using either a surgical incision and a venotomy or percutaneously using a wire introducer (Seldinger technique). The catheter is connected to a small chamber (port) that is inserted subcutaneously. After the incision is closed, a hypodermic needle can easily penetrate the chamber by percutaneous puncture. There are several publications in the veterinary literature identifying complications of vascular access ports, including seroma formation, catheter blockage, infection, and incision dehiscence, but the complication rate is low (Culp et al., 2010).
Infusion apparatus
Fluid for IV therapy is transferred from plastic bags or bottles, 500 mL up to 10 L, through tubing known as ‘administration sets’. Essentially, an administration set consists of a sharp rigid plastic end, that may or may not include an air inlet with its own filter, that is inserted into the outlet of the fluid container, a drip chamber, a filter in sets used for blood products, and a length of tubing that leads to the venous catheter. Most administration sets also have one or more rubber-capped injection sites on the tubing. Some administration sets have two or four bag attachments for fluid loading, prolonged administration, or use in large animals. The inlet to the drip chamber from the fluid bag is available in different diameters to control the number of drops in 1 mL of fluid, for example, paediatric sets deliver 60 drops/mL and adult sets deliver either 15 or 10 drops/mL. The flow rate is controlled by means of a roller clamp and can be calculated from the number of drops that pass through the drip chamber in one minute (Box 10.2). Administration sets that have a 150 mL container ‘dosage burette’ proximal to the drip chamber can be used to control the fluid volume delivered to small patients. A volume of fluid, for example 20 mL, is let into the burette and the inlet manually closed. The rate of infusion of the fixed volume is adjusted (60 drops/mL) but when the chamber is empty a flap valve closes and shuts off the administration set.
When fluids are administered under the influence of gravity, the speed of infusion depends more on the cross-sectional diameter of the needle or catheter than on the pressure (height of the fluid container above the needle or catheter). Doubling the diameter of the needle or catheter results in a 16-fold increase in the flow rate, whereas a fourfold increase in pressure is required to double the flow rate. In circumstances where the maximum size of the catheter is limited, the flow rate can be increased by pressurizing the system. Bottles can be pressurized by pumping air through the air inlet. This procedure carries a high risk of producing air embolism if the supply of fluid runs out, so it should be used with caution and the infusion should not be left unattended. Pressure can be applied to plastic bags of fluid by manually squeezing them or by placing them within a plastic or fabric bag that can be inflated by pumping in air (pressure infuser). Air embolism can only occur with the latter system if the fluid bag contains air. Air embolism should not occur when fluid is administered by gravity flow through an administration set.
Accurate control of infusion rate is possible using an electronic infusion pump attached to the administration set (Fig. 10.5). These pumps control flow rate in several ways but commonly by applying an intermittent interruption of flow through the infusion line based on the parameters manually entered into the pump. The pumps can be programmed to deliver a set volume per hour and may display the total volume delivered and the volume remaining. Infusion pumps that control flow rate by monitoring the drip chamber are rendered less accurate because they cannot compensate for variations in drop size. Some pumps require use of specially designed administration sets that are substantially more costly than standard administration sets.
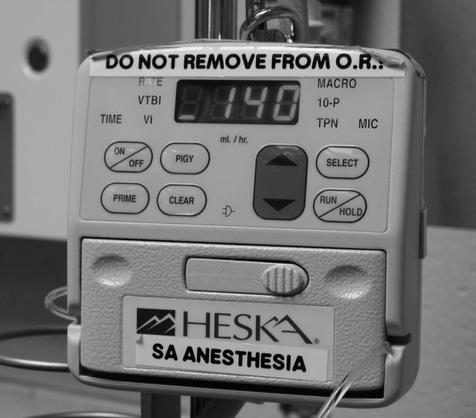
Figure 10.5 Volumetric or constant infusion pump. Devices may warn of the presence of air or occlusion of the infusion line.
Electrically driven syringe drivers (syringe pumps) are useful for accurate administration of small volumes, such as crystalloid or colloid solutions to small patients or anaesthetic agents, such as propofol, fentanyl, or lidocaine (Fig. 10.6). Extension sets between the syringe and the patient can be regular IV extensions with an internal volume of 3 mL/30 cm (12 inches) of line, or microextension sets of 1.7 metres (5 feet) with an internal volume of 0.3 mL. The device may require manual entry of the manufacturer or size of the syringe as well as the volume to be delivered per hour and the volume limit. The cross-sectional diameter of different syringes is preprogrammed in the device to allow accuracy of volume delivery. The barrel of the syringe is stationary and the unit controls the rate of plunger travel to ensure an accurate volume delivery.

Figure 10.6 Syringe driver (syringe pump). Many types of electrically driven syringe drivers are available. Some must be used with a specified size of syringe, others can be used with a variety of syringe sizes.
Although many infusion pumps have audible alarms that indicate line occlusion, infusion into perivascular tissues may not achieve a high enough pressure to trigger the occlusion alarm. Thus, the site of catheter placement must be regularly checked to confirm that fluid is being delivered intravenously and not subcutaneously or into surrounding bandage or drapes.
The infusion pump rate may be changed manually to adjust administration of anaesthetic agents. A target controlled infusion pump (TCI) is available for administration of propofol where the anaesthetist sets a target blood or effect-site concentration and the computerized infusion device makes the necessary changes to the infusion rate (Chapter 1). This device is expensive and the benefits of TCI over manual control of propofol administration have not yet been confirmed (Leslie et al., 2008). Nonetheless, protocols are being developed for use of this modality in veterinary patients.
Administration of inhalation agents
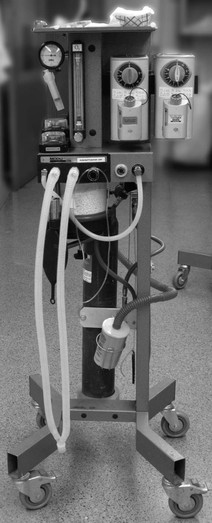
The anaesthesia machine can be complex or simple in appearance (Fig. 10.7). The essential components are the same in all machines: the anaesthesia workstation and a patient delivery circuit through which oxygen (O2) and anaesthetic gas are delivered to the patient via a mask or tracheal tube.
Anaesthesia workstation
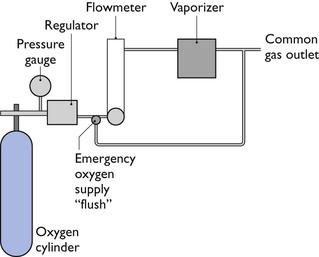
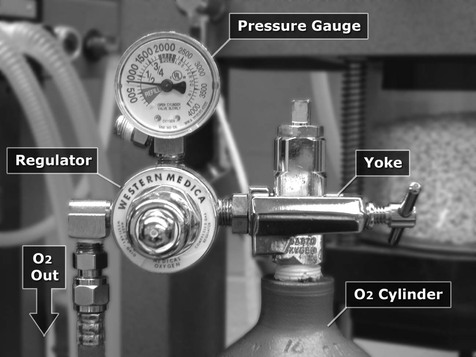
The anaesthesia workstation comprises an O2 source, a pressure regulator (reducing valve), a flowmeter, and a vaporizer(s). In addition, a pressure gauge measures pressure at the O2 source, and an emergency ‘flush’ valve will deliver O2 directly from the outlet of the regulator to the patient breathing circuit (Fig. 10.8).
Gases and cylinders
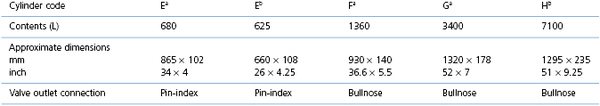
There are many different sizes of cylinders (also known as tanks) for different gases. Each cylinder is coded by a letter of the alphabet, and the type of gas by the colour of the cylinder (Table 10.1), although availability of cylinder sizes differs between countries and colour coding of the cylinders is not universal (see later). A new standard governing the colour coding of transportable gas cylinders is in transition in Europe. Designed to improve safety standards, the cylinder shoulders (the curved part at the top of the cylinder) are painted to warn of potential hazards: bright green is an inert gas, light blue is an oxidizing gas, yellow is a toxic gas, red is flammable, and white is oxygen. The product label must always be checked to identify the cylinder contents and the medical gas cylinders have distinctive colouring.
Medical grade oxygen cylinders are colour-coded white in Europe and Canada and green in the USA. The cylinders contain compressed oxygen to a pressure of 2000–2200 psi (pounds per square inch) (USA) or 137 bar (UK) when full. Oxygen in the cylinder is in gaseous phase and the cylinder pressure decreases linearly as oxygen is used. Consequently, a half-full cylinder has a pressure of half the full pressure. Observation of the pressure gauge on the cylinder and knowledge of the cylinder capacity permits an easy calculation of the current contents. Veterinary hospitals with high O2 usage may have a series of several large O2 (or nitrous oxide) H cylinders that are attached to a manifold and automatically switch from one cylinder to another cylinder as the contents are depleted, or from one series (bank) of cylinders to another.
Bulk oxygen supply in the form of a cylinder of liquid oxygen is often chosen by large hospitals. Liquid oxygen is stored in a cryogenic cylinder that is an insulated, vacuum-jacketed, pressure container equipped with pressure-relief valves and rupture discs to protect the cylinder from increased pressure. The capacity of the cylinder is between 80 and 450 L of liquid oxygen at a pressure of 350 psi and, at 20°C (68°F), the expansion ratio, liquid to gas, is 1 to 860. Strict adherence to safety protocols is essential to avoid personal injury when handling the storage cylinder.
Oxygen generators that produce a continuous supply of medical grade 93% O2 are available for veterinary practice. Convenience, safety, and decreased cost per litre are suggested reasons for using a generator, but it is essential to be certain that O2 can be generated at a sufficient rate for an emergency situation. Oxygen generators are available in the USA that can generate sufficient O2 for those practices that have multiple anaesthesia machines and utilize oxygen cages, mechanical ventilators, or offer hyperbaric chamber O2 therapy.
Nitrous oxide (N2O) is available in cylinders that are colour-coded blue or, in Europe, with a dark blue shoulder and white body. During the filling process, N2O is compressed to a liquid so that a full cylinder is approximately one-third liquid and two-thirds gas and exerts a pressure of approximately 760 psi (USA) or 44 bar (UK). As N2O is used during anaesthesia, liquid is vaporized to replace the lost gas and pressure is maintained constant. Use of a high flow of N2O may be accompanied by formation of ice crystals on the outside of the cylinder and a slight fall in cylinder pressure; when the cylinder rewarms to room temperature, the cylinder pressure returns to 760 psi or 44 bar if there is any liquid remaining. Consequently, the pressure gauge cannot be used to determine the volume of contents, as all the liquid is not vaporized until the cylinder is approximately 80% used, at which point the pressure reading decreases. Nitrous oxide is also available in different sizes of cylinders.
Compressed medical grade air containing 21% oxygen is available in cylinders for use blended with oxygen for long-term ventilation of dogs and cats in the Intensive Care Unit or for inhalation anaesthesia. In Europe, the colour code of medical grade air is a white and black shoulder with a white body.
Carbon dioxide (CO2) is available in cylinders colour-coded grey or a grey shoulder with a white body. Currently, CO2 is more commonly used in veterinary practice independently of the anaesthesia machine to produce pneumoperitoneum during laparoscopy.
Helium–oxygen (Heliox) 65 : 35, 70 : 30, or 80 : 20 mixtures are available in cylinders for special anaesthesia circumstances, such as anaesthesia or ventilation of people with respiratory distress from airway obstruction where turbulence of airflow in the trachea or bronchi produces resistance to breathing. The density of helium is lower than oxygen and the lighter helium–oxygen mixture tends to maintain laminar flow in the airways, increase flow rate, and decrease work of breathing. The improved airflow in and out of the lungs may support improved blood gases. Use of helium in anaesthesia is not widespread as although there are published case reports in human literature supporting beneficial effects of helium use, studies in larger groups of patients are not conclusive (Harris & Barnes, 2008; Maggiore et al., 2010). The pressure in a full cylinder is 2000 psi (USA) or 137 bar (UK).
Pressure gauges
Cylinders are connected to pressure gauges that register the pressure of gas within the cylinder from which the anaesthetist may be able to determine the volume of cylinder contents (Fig. 10.9). Pipeline pressure gauges must also be installed close to the locations of use when the gas supply cylinders are situated at a distance.
Regulators
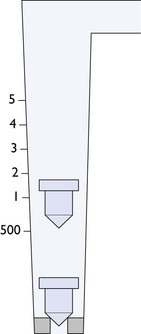
Delivery of pressure as high as in the O2 cylinder directly to the patient will cause lung damage and, therefore, a regulator (also known as a reducing valve) is necessary to decrease the pressure to a safer workable pressure. The regulator decreases the pressure to 50 psi (4 bar, 350 kPa) and maintains the outlet pressure constant for flows up to 15 L/min. Further, the outlet pressure is maintained as the pressure decreases inside the cylinder. The regulator attachment (yoke) that is placed around a pin-index cylinder head (see Fig. 10.9) or into the outlet of larger cylinders is designed to fit only a cylinder of a specific gas. Where large O2 cylinders are situated away from the operating room, regulators, similar to those on the simple workstation, reduce the pressure to a working level. Gas is then transported from the remote site through pipes to the hospital rooms where they exit the walls or ceilings. Safety features to prevent mixing gas supplies are in the form of different sized screw threads for attachment of hoses on the anaesthesia machines or by quick-connect couplings with different configurations. A pressure drop along the pipes generally occurs, consequently, the origin outlet pressure must be adjusted to maintain the pipeline outlet pressure at slightly higher than the workstation regulator outlet pressure. Some large animal mechanical ventilators are unable to function properly if the O2 pressure decreases.
Flowmeters
Gas flows to the patient breathing circuit are controlled by flowmeters. These are calibrated for each gas in mL/min or L/min as the density and viscosity of each gas determines rate of flow (Fig. 10.10). The calibrations range from 10 mL/min to 10 L/min for small animals and up to 10 or 15 L/min for large animals. The rotameter type of flowmeter consists of a glass tube inside which a rotating bobbin is free to move up and down, allowing gas to flow around it (Fig. 10.11). The tube is tapered with the diameter of the tube gradually increasing from the bottom to the top so that the annular space between the tube and rotameter (orifice) becomes wider as the rotameter rises in the tube, allowing the flow of gas to increase. The rotameter has an upper rim that is of a diameter slightly greater than that of the body, and in which specially shaped channels are cut. The gas flowing through the channels causes the rotameter to spin with the result that it rides on a cushion of gas thereby eliminating errors due to friction between the tube and the bobbin. Flow is read accurately from the top of the rotameter against a scale etched on the outside of the glass tube. Flow is accurate (± 2%) for the calibrated gas if the flowmeter (anaesthesia machine) is absolutely upright.

Figure 10.10 Oxygen and nitrous oxide flowmeters each have two tubes on this anaesthesia machine, one is expanded flow 0–1 L/min for accuracy and the other 1–10 L/min. Gas flow is read from the top of the rotameter. The air flowmeter is a single tube.
Ball flowmeters, like the rotameter, are also tapered and are, therefore, variable orifice meters. Flow rate is read from the middle of a ball when there is only one, or when there are two balls, from the point of contact between the two balls.
Vaporizers
Vaporizers for volatile liquid anaesthetic agents consist of a chamber through which O2 flows and carries anaesthetic molecules out to the patient. In the early days of anaesthesia, vaporizers were simple and subject to changes in output according to the gas flow, temperature of the liquid, surface area in contact with O2, and pressure changes generated by artificial ventilation. Simple vaporizers are used today in specific circuits that involve the patient breathing through them (draw-over) such as the Komesaroff or Stephens machines. More commonly, vaporizers have been designed to compensate for extraneous factors (precision vaporizers) and accurately deliver the dialled concentration (Fig. 10.12). The surface area is standardized by use of a wick inside the vaporizer and temperature changes are compensated for by mechanisms that alter either the inflow or outflow of O2 through the vaporizing chamber. As vaporizers are designed for use in human anaesthesia, the accuracy of the vaporizer is guaranteed only over a very limited temperature range close to 20°C (68°F). The actual specification depends on the make and model but many vaporizers may be unable to compensate for low operating room temperatures of around 17°C (63°F) resulting in low anaesthetic output and inadequate anaesthetic depth. Even more dangerous is the potential for higher than expected concentrations when working at high ambient temperatures. The anaesthetic output decreased progressively during high O2 flow rates (10 L/min) and to a greater extent from some sevoflurane vaporizers (exceeding 20% of the dial setting) than from isoflurane vaporizers (Ambrisko & Klide, 2011). The output concentration from sevoflurane vaporizers that were only partially filled was similar to that when they were full. Supplying heat by wrapping the vaporizer in a warm cloth will increase anaesthetic output but can result in an excessive depth of anaesthesia. A check valve on the outlet side of the vaporizer is used to prevent back-pressure from the patient circuit during artificial ventilation altering vaporization and output concentration. The output from precision vaporizers must be measured for accuracy at regular intervals which, in some countries, may be a specific time interval.

Figure 10.12 Precision vaporizers for isoflurane and sevoflurane. The dials indicate % output of each vaporizer. The handwritten labels below the dials note the actual % delivered at each setting at the time of the last scheduled machine check.
The maximum concentration of an anaesthetic agent at a given temperature depends on the vapour pressure of the agent. Isoflurane and sevoflurane have sufficiently different vapour pressures that they must be used in specifically manufactured vaporizers. The vapour pressure of isoflurane is similar to that of halothane, an older agent since discontinued in many countries, and halothane vaporizers can be serviced professionally, cleaned and recalibrated for use with isoflurane.
The fluid level in the vaporizer can be checked through a clear window on the side of the vaporizer. Filling of vaporizers is invariably accompanied by loss of anaesthetic vapour into the room. An inexpensive non-disposable filling spout can be attached to the bottle of isoflurane or sevoflurane and will minimize spilling. New vaporizers have ‘keyed’ filling ports where the bottle of anaesthetic agent is attached directly to the vaporizer or by using an adapter that is totally closed.
Desflurane is a colourless liquid below a temperature of 22.8°C. It requires an expensive specialized vaporizer in which the chamber holding the liquid is heated electrically to above boiling point. The vaporizer is a dual gas blender (O2 and desflurane vapour) with the output from the vaporization chamber pressure regulated to deliver an accurate percentage to the patient circuit. Special care must be taken to follow manufacturer’s instructions when filling a desflurane vaporizer as a number of potentially harmful accidents have been reported.
A number of devices, such as the ‘Selectatec’, are available to enable vaporizers to be easily attached to or removed from the ‘back bar’ of the workstation, allowing convenient exchange of vaporizers for changing anaesthetic agent, for removal for filling elsewhere, and when a vaporizer has to be sent away for service. Tipping the vaporizer when the dial is not switched off (either when moving the vaporizer or rocking the anaesthesia machine) may result in a surge of high anaesthetic concentration when the vaporizer is first turned on. To avoid accidental overdose, it is advisable to flush the vaporizer and hoses with oxygen before connecting to a patient.
Simple, low internal resistance, draw-over vaporizers for isoflurane or sevoflurane are situated within the circle delivery circuits of the Komesaroff and Stephens anaesthesia machines. The dials of the vaporizers are not calibrated and simply vary the proportion of gas passing through the vaporizers. The outputs of the vaporizers are largely determined by the magnitude of the patient’s ventilation that flows through the circuit.
Oxygen flush valve
This valve is situated between the regulator and the flowmeter. Activation causes a flow of oxygen at a pressure of 55 psi (400 kPa) and 60 L/min to the delivery circuit, by-passing the precision vaporizer(s). The flush valve should never be employed for a non-rebreathing circuit with a 0.5 L bag when an animal is attached because of the high risk of rupturing the lungs. With a small animal circle circuit, depending on the duration of flush and the size of the rebreathing bag, use of the flush valve may not be fatal. Two consequences will occur: pressure within the circle increases and the patient’s lungs will be inflated; and secondly, O2 without anaesthetic agent is delivered and thus the circle anaesthetic concentration will decrease. If the depth of anaesthesia is too light, operating the flush valve to fill the reservoir bag will further lighten anaesthesia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree