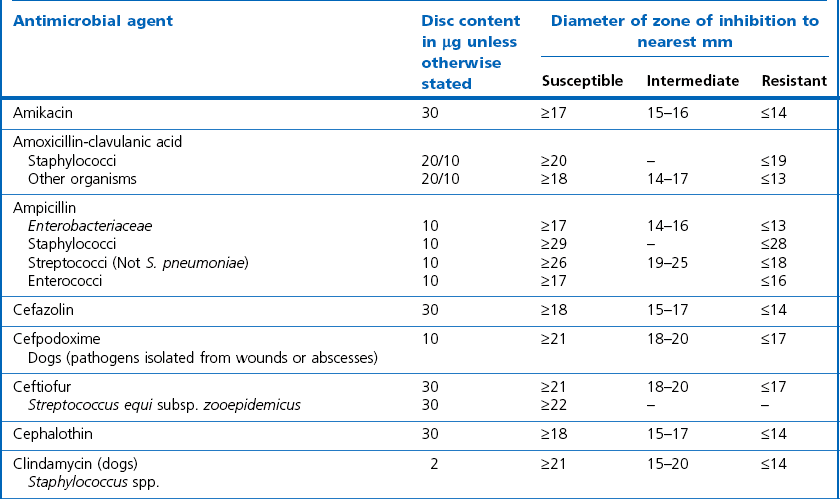
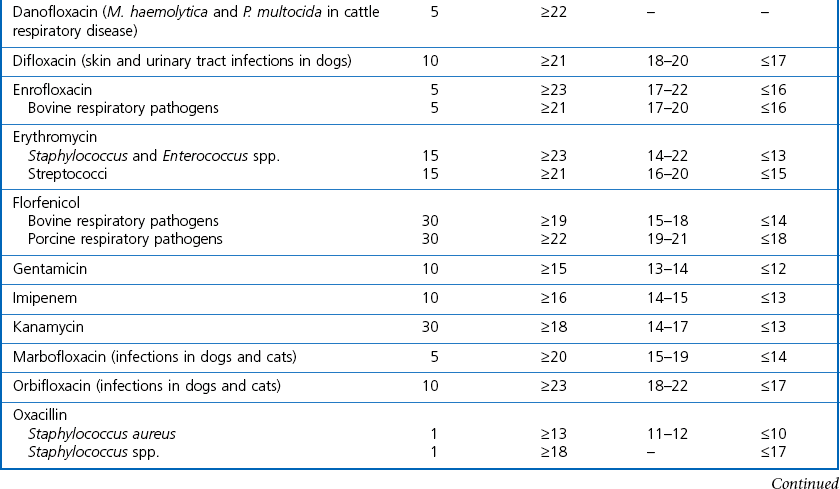
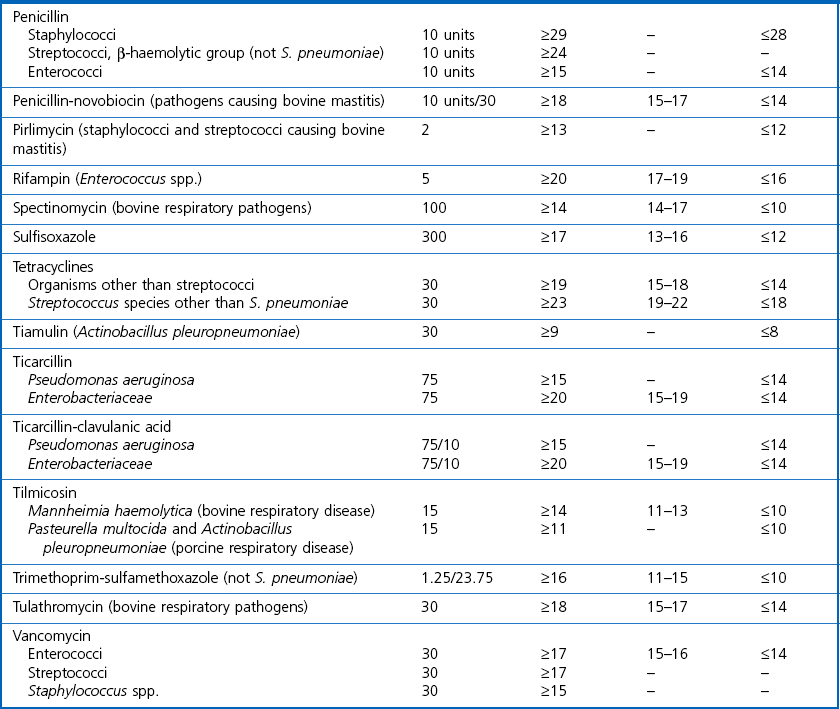
Chapter 6 Disc diffusion is the simplest method to perform. It entails the placing of discs impregnated with antimicrobial agents onto an agar plate seeded with the bacterium to be tested. The antimicrobial agents diffuse into the agar creating a zone saturated with the agent, in which an organism susceptible to that agent will not grow. The edges of the zone are the point of minimum inhibitory concentration (MIC), however, due to inconsistent rates of dispersion an accurate MIC cannot be determined by this method. The method is simple to perform, reproducible and low cost to run. The method should only be used for the rapidly growing pathogens. In order for the results to be clinically reliable the technique must be carried out in a standardized manner. Standards for the performance of this test method have been described, with many laboratories following the methods described by the NCCLS, now renamed the Clinical and Laboratory Standards Institute (CLSI). The standard developed for testing of bacteria isolated from animals is described in document M31-A3 (CLSI, 2008). • The bacterial concentration of the inoculum: this is of great importance and is usually addressed by ensuring the turbidity of the inoculum is adjusted to a 0.5 McFarland opacity standard. The aim is to have a dense lawn of bacterial growth with the individual colonies just touching each other (Figs 6.1 and 6.2). Figure 6.1 Staphylococcus aureus on Iso-Sensitest agar indicating the effect of a correct inoculum (left) and a heavy inoculum (right) on the size of the zone of inhibition. Figure 6.2 Staphylococcus aureus on Iso-Sensitest agar tested against gentamicin (CN), enrofloxacin (ENO), chloramphenicol (C) and tetracycline (TE) and illustrating an inoculum size that gives a lawn of the correct density. • The test medium: Mueller–Hinton or modifications of this medium (Iso-sensitest agar, Oxoid) is usually chosen for routine susceptibility tests. It gives good batch-to-batch reproducibility; is low in sulphonamide, trimethoprim and tetracycline inhibitors; and most pathogens grow satisfactorily on the medium. However, quality control checks should be carried out on each new batch of medium. • Excessive amounts of thymidine or thymine in a test medium can inhibit sulphonamides and trimethoprim, yielding smaller zones or no zones at all. Variation in divalent cations, mainly magnesium and calcium, will affect the zone size of tetracycline, polymyxin and aminoglycoside tests against Pseudomonas aeruginosa. • For bacteria, such as streptococci, that are unable to grow on Mueller–Hinton agar, blood agar with 5–10% defibrinated sheep blood can be used. However, the zone size, particularly for nafcillin, novobiocin and meticillin, will be 2–3 mm smaller than the normal control limits (Figs 6.3 and 6.4). Figure 6.3 Staphylococcus aureus on Isosensitest agar tested against novobiocin (NO), trimethoprim-sulfamethoxazole (SXT) and sulfafurazole (SF). Compare with Figure 6.4 when blood agar is used. Figure 6.4 The same isolate of S. aureus as in Figure 6.3 on blood agar tested against novobiocin (NO), trimethoprim-sulfamethoxazole (SXT) and sulfafurazole (SF). Note the smaller zone sizes compared with Figure 6.3. • The depth of the agar and the pH of the medium must be standardized as these may also have an effect on the zone size (see Quality Control Methods). • The antimicrobial agent and its concentration in the disc: the ability of the agent to diffuse through the agar varies and the zone of inhibition for some drugs, such as streptomycin, is always comparatively small. The concentrations of the antimicrobial agents in the discs have been chosen to give zone sizes that correlate with achievable serum levels in the patient. The zone sizes and their interpretation are given in Table 6.1. At present there are insufficient data for the correlation of in vitro tests and the clinical use of topical agents for skin, eye and ear conditions. The potency of the agent in the discs must be maintained and storage and handling methods are discussed in Quality Control Procedures. • Incubation conditions: these have been standardized for routine susceptibility tests to aerobic incubation at 35°C for 16–18 hours and 24 hours for the staphylococci. The plates must not be incubated under an increased concentration of carbon dioxide as this can significantly alter the zones of inhibition with some antimicrobial agents. A modified method is used for Haemophilus species and other fastidious bacteria that require carbon dioxide for growth (CLSI 2012, M02-A11). Anaerobes should not be tested by the disc diffusion method and the CLSI (2012) has published a standard method for testing the susceptibility of anaerobes (document M11-A8). • The test bacterium: the degree of resistance or susceptibility of a bacterium to selected antimicrobial agents will vary with the species and strain of the pathogen. There are specific difficulties in detecting meticillin-resistant staphylococci (see below). • At least four to five well-isolated colonies of the same morphological type are selected from a non-selective agar plate. Just the tops of the colonies are touched and the growth transferred to a tube containing 4–5 mL of soybean-casein digest broth or an equivalent (tryptone soya broth, Oxoid). • The inoculated broth is incubated at 35–37°C until a slight visible turbidity appears, this is usually within two to eight hours. • The colonies are selected as before and a suspension is made in saline or broth without the preincubation in broth. This is suggested as the best method for testing staphylococci, especially with suspected meticillin-resistant strains. • The turbidity of both the pre-incubated broth and the suspension of bacteria (alternative method) is adjusted by comparison with a 0.5 McFarland turbidity standard. The standard and the test suspension are placed in similar 4–6 mL thin-glass tubes or vials. The turbidity of the test suspension is adjusted with broth or saline and compared with the turbidity standard, against a white background with contrasting black lines until the turbidity of the test suspension equates to that of the turbidity standard. Alternatively, turbidity can be measured using an instrument such as a Densimat (bioMérieux). • A sterile, non-toxic swab on an applicator stick is dipped into the standardized suspension of bacteria and excess fluid is expressed by pressing and rotating the swab firmly against the inside of the tube above the fluid level. • The swab is streaked in three directions over the entire surface of the agar with the objective of obtaining a uniform inoculation. A final sweep with the swab can be made against the agar around the rim of the Petri dish. • The test agar must be Mueller–Hinton agar or a satisfactory equivalent such as Iso-sensitest agar (Oxoid). The exception is the use of 5–10% sheep blood agar for streptococci or Trueperella (Arcanobacterium) pyogenes that are unable to grow on Mueller–Hinton agar. The surface of the agar should be moist but no droplets of moisture should be visible on the surface of the agar. • The inoculated plates are allowed to stand for three to five minutes, but no longer than 15 minutes, for any excess moisture from the inoculum to be absorbed by the agar before applying the antimicrobial discs. • The discs are placed onto the agar surface using sterile forceps or an antibiotic disc dispenser (Fig. 6.5). Each disc is gently pressed with the point of sterile forceps to ensure complete contact with the agar surface. The discs should be placed no closer together than 24 mm (centre-to-centre). This is equivalent to six discs per standard 90 mm Petri dish. Figure 6.5 The materials required for susceptibility testing showing an antibiotic disc dispenser for the correct spacing of discs on the lawn of the test bacterium. • The plates are inverted and placed in a 35°C incubator, within 15 minutes of applying the discs and incubated aerobically for 16–18 hours or 24 hours for staphylococci. • After incubation the diameters of the zones of inhibition are measured to the nearest mm using a ruler or calipers. The diameters are read from the back of the plate when the test is on the comparatively clear Mueller–Hinton medium but over the surface of the agar with streptococci grown on blood agar. The diameter of the zones should be read across the centre of the discs. Automated devices for reading and interpretation of zone sizes are available (e.g,. Mastascan, Mast Group Ltd.). An interpretation of the size of the zones of inhibition is made with reference to available interpretative tables such as those produced by CLSI. Table 6.1 is modified from CLSI (2008) document M31-A3. The bacterium is reported as susceptible, intermediately susceptible or resistant to each antimicrobial agent used in the test: 1. Large colonies growing within an otherwise clear zone of inhibition should be subcultured, re-identified and retested. Faint growth of tiny colonies at the edge of the zone can be ignored. 2. Proteus species may swarm into areas of inhibited growth around certain antimicrobial discs. If the zones of inhibition are clearly outlined then the veil of swarming can be disregarded. 3. With trimethoprim and the sulphonamides, slight growth in the zone can be ignored and the zone measured to the margin of heavy growth. 4. For bacteria that have to be grown on blood agar plates, the zone size for nafcillin, novobiocin, oxacillin and meticillin will be 2–3 mm smaller than the normal control limits. 5. Transmitted light should be used to examine for light growth within the zone in the case of meticillin-resistant strains of staphylococci. • An oxacillin disc is used because it is more stable in storage and is more likely to detect cross-resistance than a meticillin disc itself. • The alternative method of standardizing the inoculum, without pre-incubation in broth, is preferred for testing staphylococci. • If the incubator cannot be controlled at 35°C, then a separate test should be carried out with oxacillin and incubated at 30°C. Resistance to meticillin or oxacillin may not be seen at 37°C. • Meticillin-resistant strains often have multiple resistance that includes the beta-lactams, aminoglycosides, marcrolides, clindamycin and tetracyclines. This may act as a clue to resistance to penicillinase-resistant penicillins. • A film of growth within a zone of inhibition around a meticillin, oxacillin, nafcillin or cephalothin disc also indicates heteroresistance. • Confirmation of intrinsic oxacillin-resistance in staphylococci can be made by inoculating the suspect strain onto a quadrant of Mueller–Hinton agar that has been supplemented with 4% NaC1 and 6 µg oxacillin per ml. The inoculation is made with a suspension adjusted to the 0.5 McFarland standard. The plate is incubated at 35°C for 24 hours. Any growth indicates intrinsic oxacillin-resistance. • A tetracycline disc will predict the result against all the other tetracyclines • Sulfisoxazole is a suitable representative for all the sulphonamides • Erythromycin is the only macrolide that requires testing • A clindamycin disc will predict the result for lincomycin • Any aminoglycosides or quinolones required clinically should be tested separately as often the drugs are not related closely enough to assume cross-resistance • Chloramphenicol, vancomycin, nitrofurantoin and trimethoprim/sulfamethoxazole are tested separately as required.
Antimicrobial agents
Antimicrobial susceptibility testing
Disc Diffusion Method
Factors affecting the size of the zone of inhibition
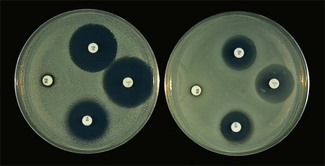
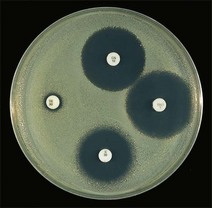
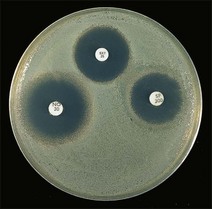
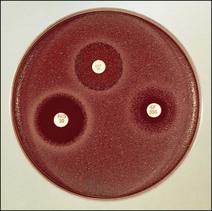
Routine test procedure for the disc diffusion method
Standard method
Alternative method
McFarland 0.5 turbidity standard

 Susceptible: the infection may respond to the treatment at the normal dosage.
Susceptible: the infection may respond to the treatment at the normal dosage.
 Intermediate: this category may be used when the pathogen may be inhibited by attainable concentrations of the antimicrobial agent provided a higher dosage is used or the pathogen is in a certain body site, such as urine, where the drug is physiologically concentrated. Classification of a result as intermediate can be used for technical reasons also.
Intermediate: this category may be used when the pathogen may be inhibited by attainable concentrations of the antimicrobial agent provided a higher dosage is used or the pathogen is in a certain body site, such as urine, where the drug is physiologically concentrated. Classification of a result as intermediate can be used for technical reasons also.
 Resistant: the bacterium is not inhibited by the usually achievable systemic concentrations of the antimicrobial agent and efficacy has not been reliable in clinical studies.
Resistant: the bacterium is not inhibited by the usually achievable systemic concentrations of the antimicrobial agent and efficacy has not been reliable in clinical studies.
Some observations on the interpretation of zones of inhibition
Meticillin-resistant staphylococci
Selection of antimicrobial discs
 Staphylococci should be tested against penicillin G and oxacillin (for resistance to meticillin, cephalosporins and other betalactams)
Staphylococci should be tested against penicillin G and oxacillin (for resistance to meticillin, cephalosporins and other betalactams)
 Enterococcus faecalis tested against penicillin G will predict the result against ampicillin, ampicillin-analogues, amoxicillin and the acylamino-penicillins
Enterococcus faecalis tested against penicillin G will predict the result against ampicillin, ampicillin-analogues, amoxicillin and the acylamino-penicillins
 Streptococci should be tested against either penicillin G or ampicillin, testing against both is unnecessary.
Streptococci should be tested against either penicillin G or ampicillin, testing against both is unnecessary.
 Staphylococci are usually susceptible except for the meticillin-resistant strains. These should be reported as resistant even if the result of the in vitro test suggests otherwise.
Staphylococci are usually susceptible except for the meticillin-resistant strains. These should be reported as resistant even if the result of the in vitro test suggests otherwise.
 There is a lack of clinical correlation with Enterococcus faecalis against the cephalosporins and the results of the in vitro tests cannot be interpreted with accuracy.
There is a lack of clinical correlation with Enterococcus faecalis against the cephalosporins and the results of the in vitro tests cannot be interpreted with accuracy.
 A cephalothin disc will represent the result to cefaclor, cephapirin, cefazolin, cephradine, cephalexin and cefadroxil. Cefatozime represents ceftazidime, ceftizoxime and ceftriaxone.
A cephalothin disc will represent the result to cefaclor, cephapirin, cefazolin, cephradine, cephalexin and cefadroxil. Cefatozime represents ceftazidime, ceftizoxime and ceftriaxone.![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Antimicrobial agents
Only gold members can continue reading. Log In or Register to continue