Type of model
Model description
T cell mediated response
Advantages and disadvantages
1. EAU induced animal models of uveitis
A. “Classical” EAU: immunization of wiId-type mice with an ocular antigen in CFA.
Also, can adoptively transfer immune cells or pathogenic cell line from immunized donors to naive recipients
EAU induced in B10.RIII, B10.A and C57BL/6 mice with IRBP
Classical EAU is Th17 driven
Adoptive transfer of polarized retina specific effector T cells is Th1 or Th17 driven
Mycobacteria in CFA provide innate “danger” signals that can polarize autoimmune lymphocytes towards a pro-inflammatory phenotype. Pertussis toxin (PTX) is needed as an additional adjuvant in less susceptible strains.
Model is highly reproducible and provides consistent disease similar to human disease. Mice can be used in preclinical studies and testing of potential therapeutic agents
B. Immunization of rats with retinal antigen in CFA or adoptive transfer of T cells from immunized donors to naive recipients
Arrestin-induced model in the Lewis rat.
Other retinal and choroidal (melanin) antigens are uveitogenic in Lewis rats
Th17 driven
Was the major EAU model until 1988. Currently, it is not often used
C. EAU by infusion of antigen-pulsed syngenic dendritic cells (DCs)
B10.RIII mice are given splenic DCs elicited with Flt3, matured in vitro, and pulsed with IRBP p 161–180
DC-EAU is Th1 driven
Requires two injections of DCs and pertussis toxin. Less severe disease than CFA-EAU and depends mainly on Th1 cells
D. Neo-self-antigen expressed in the mouse eye transgenically under a retinal promoter or by retroviral transduction following intraocular injection. Mice are then immunized with the specific antigen
Transgenic HEL or β-gal under the control of an eye-specific promoter or can be retrovirally introduced influenza hemagglutin (HA) in the retina
Th1 driven
Uveitogenic T cells need to be activated
2. Humanized animal models of uveitis
A. “Humanized” EAU: HLA-DR3 transgenic mice are immunized with an ocular antigen or adoptively transferred with T cells from immunized donors
Induced EAU in HLA-DR3 transgenic mice immunized with retinal arrestin or its peptide fragments
Not well characterized
Mycobacteria in CFA provide innate “danger” signals that can polarize autoimmune lymphocytes towards a pro-inflammatory phenotype
B. Mice transgenic for a human HLA class I antigen associated with uveitis
HLA-A29 transgenic mice. Birdshot retinochoroidopathy like disease develop between 8–12 months of age
Not well characterized
Mice require “aging” for disease to manifest. Model is clinically relevant
3. Spontaneous animal models of uveitis
A. AIRE knockout mice
Spontenous uveitis occurs in AIRE-deficient mice directed at IRBP
Not well characterized
High frequency and affinity of cells bear TCRs specific for IRBP. May not represent the physiological state found in humans. Not much is known about the effector T cell type.
B. EAU in IRBP TCR trangenic mice
Spontaneous uveitis occurs in mice by weaning age. There are three lines with high (R161H), medium (R161M), and low (R161L) disease
EAU in R161 mice is Th1 driven
Mice can be used to study natural triggers of uveitis. There is no need for CFA or an adjuvant. Preclinical studies can be done in the three different lines, which produce different severities of disease. Mice can develop lymphomas
C. Double-transgenic mice expressing a neo-self-antigen in the retina and an antigen-specific TCR
Spontaneous EAU-like uveitis in mice expressing ocular HEL and a HEL-specific TCR
Th1 driven
Spontaneous nature of disease in this model is dependent on the frequency and affinity of the TCR
6.4 Induced Animal Models of Uveitis
6.4.1 Experimental Autoimmune Uveitis (EAU)
Over the span of five decades, different animal models of induced uveitis have been developed. The most common model is the “classical” EAU with complete Freund’s adjuvant (CFA). This model was originally developed by Aronson et al. in 1963, in the guinea pig using homologous uveal tissue, and was subsequently adapted to the rat using photoreceptor extracts by Wacker and Kaslow in 1973. In 1981, Kozak et al. refined the rat model using a purified retinal soluble antigen (S-Ag). EAU has subsequently been induced in the rat with IRBP, RPE-65, rhodopsin, recoverin, and phosducin retinal-specific proteins (Gery et al. 1986; Caspi et al. 1988) . The rat model has shown that different retinal proteins induce disease and that the disease is T cell mediated and transferable with long-term CD4+ T cell lines specific to retinal antigens. In 1988, successful induction of EAU in mice was achieved by Caspi et al. using the whole IRBP protein (Caspi et al. 1988). S-Ag was less potent than IRBP as a pathogenic antigen in mice, and in fact, mouse strains that developed EAU after immunization with IRBP failed to do so after immunization with S-Ag. Since then, pathogenic epitopes of the IRBP protein have been characterized, such as peptides 1–20, 161–180, and 201–216 that elicit EAU in mice (Caspi 2003) . EAU can also be induced in rabbits and monkeys, which have larger eyes, making them well suited for experimental manipulation (Zeiss 2013) . The EAU model, however, is most frequently used in mice and rats (Agarwal and Caspi 2004) . Mice are particularly useful due to their immunologically and genetically well-defined nature and ease of genetic manipulability (Zeiss 2013) . There is a vast array of murine transgenic strains and knockouts for immunologically relevant genes. Therefore, the rat EAU model induced with retinal arrestin (retinal soluble antigen, S-Ag) or with IRBP and the mouse EAU model induced with IRBP are the most commonly used EAU models (Agarwal and Caspi 2004; Wildner et al. 2008; Horai and Caspi 2011) .
6.4.2 Different Uveitogenic Antigens Induce Different Variants of Uveitis
Researchers induce EAU in rodents using a single dose (50–100 μg) of soluble retinal-specific antigen like bovine IRBP (whole IRBP protein or a pathogenic IRBP peptide fragment like peptides 161–180 or 1–20 can be used) emulsified in CFA, supplemented with heat-killed mycobacteria (Caspi 2008a; Agarwal and Caspi 2004) needed to activate the innate system in order to boost the immune response. Also, injection of Bordetella pertussis toxin (PTX) as an additional adjuvant at the time of subcutaneous immunization is required in less susceptible mouse strains (Silver et al. 1999) . The adjuvant effect of PTX is complex and not completely understood. It is thought to contribute to inhibition of lymphocyte recirculation, stimulatory effects on B and T cells, and enhancement of vascular permeability and disruption of the blood–organ barrier (Caspi 2003) . Nevertheless, rodents develop intraocular inflammation 9–12 days after immunization, and the inflammation typically subsides by 21–28 days (Agarwal and Caspi 2004). The clinical course of the disease is typically monophasic and acute. Antigen dose and the site of immunization impact EAU disease severity, and the antigen can be easily increased or decreased depending on the desired disease range. Disease scores are assessed by microscopic fundus examination or histopathology using a scale of 0 (no disease) to 4 (severe disease). Table 6.2 consists of additional details on EAU scoring. Moderate to severely diseased animals can develop subretinal/chorioretinal granulomas along with retinal vasculitis, retinal and optic nerve edema, photoreceptor loss, and vitreous inflammatory cell infiltration (macrophages and lymphocytes), which are all clinical features seen in human uveitis. Figure 6.1 shows some of the pathological characteristics seen in mice with EAU. As mentioned earlier, susceptibility to disease is strain-dependent. In mice and rats, both major histocompatibility complex (MHC) and non-MHC genes have effects on disease susceptibility (Pennesi and Caspi 2002; Mattapallil et al. 2008) . MHC control is likely due to the ability to bind and present uveitogenic epitopes. The Lewis rat is the most commonly used rat strain for EAU (Wildner et al. 2008) . In the mouse, the most highly susceptible strain so far is B10.RIII, followed by B10.A strain, and then C57BL/6, which are only moderately susceptible (Agarwal and Caspi 2004; Caspi 2008b) . In this model, both IFN-γ and IL-17A producing cells are detected in inflamed eyes, but CFA EAU is prevented and reversed with anti-IL-17A antibodies, indicating its dependence on the Th17 effector lineage (Horai and Caspi 2011).
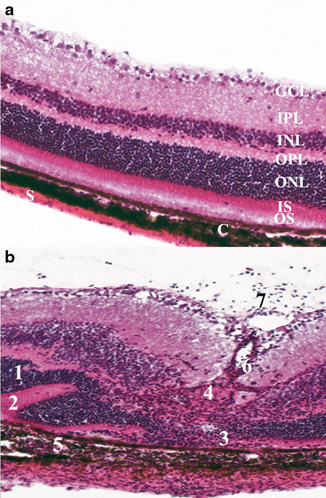

Fig. 6.1
Histopathology showing the cardinal signs of uveitis in mouse retina, which is similar to that reported in human uveitis. a Represents a normal mouse retina with all the intact retinal layers. b Shows an R161H mouse with spontaneous uveitis. There is significant inflammation and disruption of the retinal integrity. 1 Retinal folds, 2 retinal detachment, 3 photoreceptor loss, 4 granuloma formation, 5 choroiditis, 6 vasculitis (retinal vessel inflammation), 7 vitritis (a sign of active inflammatory activity). All of these pathological features can occur in varying degrees of severity and extent. GC ganglion cell layer, IPL inner plexiform layer, OPL outer plexiform layer, ONL outer nuclear layer, PR photoreceptor layer, C choroid, S sclera
Table 6.2
Scoring of EAU in the mouse by histopathology and fundoscopy
Disease score | Histopathoiogical characteristics | Fundoscopy characteristics |
|---|---|---|
0 | No disease, normal retinal structure | No change |
0.5 (trace) | Mild inflammatory cell infiltration, no tissue damage | Few peripheral focal lesions, minimal vasculitis |
1 | Infiltrations into vitreous, retina, retinal folding and vasculitis, one granuloma | Mild vasculitis, less than 5 small focal lesions |
2 | Moderate infiltration into the vitreous, uvea and retina, retinal folds and detachment, focal photoreceptor damage, small granulomas | Multiple chorioretinal lesions and/or infiltrations, severe vasculitis |
3 | Moderate-to-severe infiltration into uvea, vitreous and retina, extensive retinal folding with retinal detachment, subretinal neovascularization, medium granulomas | Large lesions, subretinal neovascularization, retinal hemorrhages |
4 | Severe infiltration, subretinal neovascularization and hemorrhages, extensive photoreceptor damage, large granulomatous lesions | Large retinal detachment, retinal atrophy |
6.4.3 Experimental Melanin Protein-Induced Uveitis (EMIU)
It is important to note that non-retinal proteins can also be used to induce uveitis in animals, examples include melanin or tyrosinase-related proteins, which are proteins in the melanin synthesis pathway, and even melanin basic protein (MBP; distinct from MBP = myelin basic protein that is used to elicit experimental autoimmune encephalomyelitis-associated anterior uveitis (EAE/AU) ahead; Chan et al. 1994; Bora et al. 1995; Commodaro et al. 2011) . Each of these antigens can induce uveitis of somewhat different form and duration. For example, non-soluble melanin proteins isolated from pigmented bovine eye tissues can induce uveitis in a model known as EMIU, which was described in the 1990s by Broekhuyse et al. (Broekhuyse et al. 1991, 1992, 1991) . This model can be induced by subcutaneous injection of melanin proteins in association with Hunter adjuvant in rats and monkeys. Ocular inflammation is evident in iris, ciliary body, and choroid. Ocular inflammation is observed 10–14 days after immunization and consists of conjunctival hyperemia, corneal edema, anterior uveitis, iris vessel dilation, and synechiae (Gasparin et al. 2012) . Both CD4+ and CD8+ T cells, macrophages, and neutrophils infiltrate into the eye. IL-2 and IFN-γ are the major intraocular cytokines produced early in the disease, while IL-12 and TNF-α play a role later in the inflammatory response (Papotto et al. 2014; Gasparin et al. 2012) . It has also been suggested that complement plays a role in the EMIU model (Jha et al. 2006) . Essentially, this model is useful for studying anterior uveitis (iridocyclitis), where melanin is found, and its spontaneous recurrence (Smith et al. 2008) . The relapsing feature of this model makes it useful for the evaluation of therapies initiated during established disease and aimed at the prevention of recurrence.
6.4.4 Experimental Autoimmune Encephalomyelitis-Associated Anterior Uveitis
Myelin basic protein can also induce inflammation in rat eyes following subcutaneous immunization with CFA (Gasparin et al. 2012). This experimental model is known as EAE/AU (Constantinescu and Lavi 2000) (Adamus et al. 1996) . Mice and rats immunized with MBP will typically experience ocular inflammation 10–12 days after immunization. Clinical symptoms include iris vessel dilation, anterior uveitis, and synechiae, which can persist for 30 days (Adamus et al. 1996). IL-2 and IFN-γ produced by Th1 cells are the predominant inflammatory mediators present in this model (Gasparin et al. 2012; Adamus et al. 1996) . This model is particularly ideal for studying anterior uveitis and the inflammatory relapses associated with this disease.
6.4.5 Endotoxin-Induced Uveitis (EIU)
Endotoxin can be used to induce ocular inflammation in a model known as EIU. This is a non-autoimmune model of uveitis. It can be induced by intravenous, intraperitoneal, or subcutaneous injection of low doses of endotoxin in mice and rats (Rosenbaum et al. 1980; Papotto et al. 2014) . In rats, macroscopically ocular inflammation can appear within 18–24 h post-LPS injection and can resolve spontaneously within 2–3 days (Papotto et al. 2014). The histopathology of EIU is characterized by transient, but intense, acute inflammatory cellular infiltration of neutrophils and macrophages, as well as protein accumulation in the anterior chamber and mild posterior uveitis (Bora et al. 1995; Broekhuyse et al. 1992) . In mice, inflammation is typically much milder and is best quantitated by cellular counts and/or measurement of cytokines in ocular fluids (Li et al. 1995) . Various cytokines and chemokines are released by infiltrating and ocular resident cells, including TNF-α, IFN-γ, TGF-β, IL-1, IL-6, CCL-2, and CCL-5 (Gasparin et al. 2012). Repeated injections of LPS can result in a state of tolerance in animals. This model is useful to study acute and subacute anterior ocular inflammation in humans.
6.4.6 Adoptive Transfer Model of T Cells from EAU Challenged Animals
Immune T cells from EAU-induced animals can transfer disease to naïve, genetically compatible recipient animals by a process known as adoptive transfer (Caspi 2003) . Primary cultures from immunized donors are used as a source of uveitogenic effector cells. T cells are isolated from lymph nodes and spleens of donors immunized with a retinal antigen, such as IRBP, and activated in vitro with the same immunizing ocular antigen (Agarwal et al. 2012; Caspi 2003) . The activated effector T cells (typically 30–50 million) are then infused intravenously into host animals after 3 days in culture (Caspi 2003). The recipients of these cells develop a destructive disease, usually within 1 week. Loss of photoreceptor cells, infiltration of inflammatory cells, and loss of vision can be seen in the eye of recipient mice. Also, long-term antigen-specific T cell lines, which are typically CD4+ T cells of the Th1 phenotype, can be derived from draining lymph node cells of IRBP or peptide-immunized mice and transferred into the recipient mice (Agarwal and Caspi 2004; Caspi 2003, 2008c, 2010, 2011) . The adoptive transfer model is useful to analyze effector mechanism(s) of disease. It avoids the use of adjuvants in the recipients; hence, it is considered by some to be more reminiscent of human clinical uveitis.
6.4.7 EAU Induced with Antigen-Pulsed Dendritic Cells (DCs)
DCs are professional antigen presenting cells (APCs) capable of stimulating naïve T cells and are likely the main APCs in the early stages of EAU induction . A new model of EAU was developed by injection of matured splenic DC loaded with the major uveitogenic peptide of IRBP into the B10.RIII strain of mice (Caspi 2008a; Tang et al. 2007) . The duration of the disease is shorter, pathology is less severe, and the inflammatory infiltrate is different from the classical EAU model (Caspi et al. 2008) . Fundus lesions are punctate, and the inflammatory infiltrate contains abundant granulocytes and relatively few mononuclear cells. Notably, EAU resulting from antigen-pulsed DCs is not only clinically distinct from classical EAU, but also driven by different effector mechanisms (Caspi 2008a). DC EAU cannot be induced by infusion of uveitogenic DC in IFN-γ deficient recipients, indicating a dependence on the Th1 effector lineage rather than Th17 like CFA EAU. This model may represent some types of uveitis that are not adequately represented by the classical EAU model and may offer insights into the heterogeneous nature of human disease (Horai and Caspi 2011) .
6.4.8 Other EAU-Like Models
Finally , neoantigens expressed in the retina through genetic engineering can serve as target antigens for uveitis. These foreign proteins include hen-egg lysozyme (HEL) or β-galactosidase (β-gal) that have been transgenically expressed in the retina or lens under the control of a tissue-specific promoter to serve as a neo-self-antigen. Gregerson et al. induced expression of the foreign protein β-galactosidase (β-gal) in the mouse retina and demonstrated that EAU can be induced by immunizing mice with β-gal. Alternatively, retinal cells can also be transduced in vivo to express a foreign-protein-like influenza hemagglutinin (HA), using a viral vector (Terrada et al. 2006) . Subsequent immunization with the neoantigen, or transfer of immune cells from genetically compatible donors, triggers disease in the eye, similarly to immunization with IRBP or adoptive transfer of IRBP-specific T cells in wild-type mice (Gregerson et al. 1999; Lai et al. 1999; Terrada et al. 2006; Lambe et al. 2007) .
6.4.9 The Future of EAU- and EAU-Like Induced Models in Translational Research
Various EAU models are used to represent human uveitic diseases of a putative autoimmune nature, such as sympathetic ophthalmia, birdshot retinochoroidopathy, and Behcet’s disease (Bodaghi and Rao 2008; Forrester et al. 2013; Nussenblatt 2002) . Although the EAU models reproduce some of the same features in human uveitis, they are not perfect. We have not definitively identified which retinal antigens are involved in human uveitis. However, the fact that several retinal proteins that elicit memory responses in uveitis patients also cause disease in animals suggests that similar mechanisms may occur in human disease. Likewise, this shows the heterogeneous nature of uveitis—both in mouse and man (Forrester et al. 2013) . These various EAU models have allowed researchers and clinicians to establish that uveitis is a CD4+ T effector cell mediated disease with either Th1- or Th17-mediated responses (Caspi 2011) . Also, the major cytokines identified in driving pro-inflammatory ocular disease processes include TNF-α, IFN-γ, IL-17, and IL-21, as well as many others (Forrester et al. 2013; Horai and Caspi 2011). The EAU model has served as an invaluable tool to evaluate novel immunotherapeutic modalities. Success in downregulating EAU in animals has often been predictive of clinical success of a given therapeutic agent in subsequent clinical trials.
Extrapolation from animal models has suggested that what triggers uveitis in humans could be an exposure to a retinal or antigenic mimic, combined with an infectious event that may provide inflammatory “danger” signals, creating conditions which lead to the development of ocular inflammation . Most EAU models of uveitis are dependent on activation of the innate immune system by use of adjuvants that activate a wide range of pathogen recognition receptors (PRRs). This raises the question of the underlying role of initial and/or persistent infection in immune-mediated uveitis (Forrester et al. 2013) . Pathogenic mechanisms such as antigenic cross-reactivity and molecular mimicry may exist with pathogenic or commensal microorganisms. In addition, microbial components may act as “endogenous” adjuvants for provoking immune reactions to altered self-antigen, suggesting that there may be a common link in the pathogenesis of infectious and noninfectious human uveitis (Forrester et al. 2013).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


