26 The number and variety of exotic animals being maintained as pets and for profit are increasing. Anesthesia for nontraditional species is accomplished for the most part by using the same techniques and drugs used in domestic animals. Because exotic species demonstrate idiosyncrasies and widely varying sensitivities to drugs, it is frequently necessary to make modifications. This chapter is an overview of the basic information needed to successfully immobilize exotic animals that are likely to be encountered by general practitioners.* I Preanesthetic considerations 1. Ensure that the client has realistic expectations 2. Detail prognosis and risks; owners may not understand that exotic animals respond differently to immobilization than domestic animals 3. Establish who will do the aftercare; owners may not be able to administer treatments to exotic pets B Reduce the animal’s stress; exotic animals have high sympathetic drive; excessive stress can induce complications that include myocardial arrhythmias, hypertension, and hyperthermia, which can result in death C Presurgical evaluation and animal selection a. Quiet observation of the animal in its cage can provide a significant amount of information (e.g., animal’s awareness of and attention to its surrounding environment, body position and posture, skin or feather condition, respiratory rate) b. Contraindications to anesthesia (1) Abnormally slow recovery rate (the amount of time an animal needs to return to normal respirations after 2 minutes of physical restraint or pursuit); a normal amount of time is less than 3 to 5 minutes (2) Shock; septicemia; acidosis (6) Animal has not fasted (if regurgitation is likely) (7) Severe weakness and central nervous system depression a. The volume of blood required for the sample should not exceed 10% of the animal’s blood volume (e.g., the blood volume of a 30 g [body weight] gerbil is 2.3 mL and 10% of this volume is 0.2 mL) Approximate Blood Volumes of Exotic Species b. Contraindications to anesthesia (1) A blood transfusion may be needed if packed cell volume is low Perilously Low Packed Cell Volumes in Exotic Animals (2) Fluids (3 to 5 mL/kg of body weight/hr) should be supplemented during surgery Perilously High Packed Cell Volumes in Exotic Animals (3) Amino acid or plasma supplementation may be needed if total protein is less than 3 g/dL; refractometers and colorimetric tests frequently used at diagnostic laboratories for mammalian blood often register falsely low proteins in nonmammalian species; the biuret method is the most accurate in avian species (4) Administer 5% dextrose or delay anesthesia if the glucose is low Perilously Low Blood Glucose Levels in Exotic Animals (5) Correct low calcium to greater than 8 mg/dL A Hypoglycemia can occur within a few hours in very small mammals Time for Preanesthetic Food Withdrawal in Exotic Animals A Hypothermia depresses the respiratory control system 1. Small animals are predisposed to hypothermia; they lose heat rapidly as a result of high surface-area-to-volume ratios 2. Ectotherms (reptiles, fish, and amphibians) do not generate their own heat; they require external heat sources to maintain their body temperature; hypothermia depresses the immune system and slows healing 3. Hypothermia can result in brain damage, shock, electrolyte imbalances, and disseminated intravascular coagulation a. Well-insulated, small animals such as rabbits, chinchillas, water birds, and birds in winter plumage are susceptible to hyperthermia b. Ungulates are prone to malignant hyperthermia; stress, high ambient temperature, and high relative humidity during immobilization increase the likelihood of hyperthermia B Methods of monitoring core body temperature (skin temperature is not reliable) C Heat sources and retaining heat A Perioperative hypovolemia must be prevented to decrease postoperative morbidity and mortality B Preheat to 80° F to 95° F (26° C to 35° C) for animals weighing less than 1 kg and for all ectotherms 4. Placing intravenous (IV) tubing in warm water bath 5. Fluid heating devices (Hotline, SIS Level 1, Inc.) C Administration (route) (Table 26-1) TABLE 26-1 Sites for Parenteral Access or Injection in Birds and Small Mammals IM, Intramuscular; IO, intraosseous; IP, intraperitoneal; IV, intravenous; SQ, subcutaneous. 1. IV or intraosseous (IO) administration is best; the humerus in all birds and the femur in many birds are pneumatic bones; placing IO catheters in these bones causes iatrogenic drowning 2. Subcutaneous (SQ) fluids (usually 5% to 10% of body weight) are given before induction of anesthesia to allow absorption and volume expansion 1. Standard maintenance: 40 to 100 mL/kg of body weight/ 24 hr; the higher end of the range is used for neonates, birds, and animals with high metabolic rates 2. Intraoperative: 3 to 5 mL/kg/hr 1. Rates less than 10 mL/hr require an IV fluid infusion pump to ensure accuracy 2. Rates of 10 to 50 mL/hr are accurately measured with inline flow controls or burette fluid chambers 3. Rates greater than 50 mL/hr are accurately measured off of most fluid containers F Fluid choice (see Chapter 16) G Blood transfusion (see Chapter 16) 1. Ethylenediamine tetra-acetic acid (EDTA) should not be used in small exotic animals to avoid hypocalcemia 2. Heparin is the anticoagulant of choice for birds 3. Birds can receive one interspecies or genera transfusion if a donor of the same species is not available, but survival of red blood cells over 24 hours is limited; intraspecific or at least intragenera transfusions are preferred A If necessary, administer antibiotics to achieve adequate serum levels before induction of anesthesia B Select appropriate preanesthetic medication TABLE 26-2 Injectable Sedative and Analgesic Drugs for Avian Use IM, Intramuscular; IV, intravenous; SQ, subcutaneous. 1. Anticholinergics are not indicated because they make respiratory secretions more viscous 2. Preanesthetics are rarely indicated if the bird is small enough to be manually restrained b. They may depress respirations c. Diazepam/midazolam is the most efficacious and safe—0.05 to 0.15 mg/kg IV, 0.2 to 0.5 mg/kg intramuscularly (IM), 7.3 mg/kg intranasally d. Detomidine 12 mg/kg intranasally e. Combination of midazolam (3.6 mg/kg), xylazine (10 mg/kg), and ketamine (40 to 50 mg/kg) intranasally 3. Because their long legs are susceptible to trauma, ratites, storks, and long-legged wading birds weighing more than 15 kg may benefit from sedation before capture and/or restraint 1. Anticholinergics make respiratory secretions more viscous 2. Preanesthetics are rarely indicated, except in large, aggressive, and/or venomous reptiles E Amphibians: preanesthetics are not routinely used F Fish: preanesthetics are not routinely used G Rodents, guinea pigs, and rabbits 1. Atropine or glycopyrrolate is useful for decreasing airway secretions and maintaining heart rate 2. Some rabbits have atropinase—use glycopyrrolate instead (see Chapter 3) 3. Acepromazine and diazepam are effective preanesthetics 4. Acepromazine is not recommended in gerbils, because it may potentiate seizures 5. Hamsters require up to 5 mg/kg of acepromazine 6. Combination of fentanyl and fluanisone (Hypnorm) produces sedation and immobilization for about 30 to 60 minutes; analgesic effects may persist 1. Anticholinergics are given as needed to maintain heart rate and decrease secretions 2. Diazepam or midazolam provides light tranquilization but no analgesia 3. Acepromazine provides light to moderate tranquilization but also no analgesia; should be avoided in hypovolemic or very sick animals 4. Xylazine or dexmedetomidine produces dose-dependent sedation, immobilization, and analgesia 5. Opioids potentiate sedation and provide analgesia; usually given in combination with other sedatives or tranquilizers 6. Ketamine produces dose-dependent sedation; muscle relaxation is poor if it is given alone 1. Preanesthetics are not routinely used 2. Anticholinergics can be used at standard domestic cat doses 1. Pulse rate and character: a decrease in heart rate to less than 80% of the stabilized rate after induction indicates that anesthesia should be lightened 2. Respiratory rate, volume, and character (e.g., apneustic) a. A decreasing respiratory rate or apneustic/erratic patterns indicate that anesthesia should be lightened b. Ectotherms and birds normally develop apnea at surgical planes of anesthesia; plan to administer positive-pressure ventilation at least two to six times per minute 1. Electrocardiogram modifications a. To protect delicate skin or to penetrate thick skin, attach clips to steel sutures or metal hub needles placed through the skin b. Attach clips to alcohol-soaked pads placed at the usual lead sites c. Wings are used for forelimb lead sites in birds d. Place one clip cranial and one caudal to the heart in legless animals a. Measures the percentage hemoglobin saturation with oxygen in addition to heart rate b. Placement: tongue, esophageal 4. Heart rate monitors must be suited for high heart rates and be able to read up to 350 beats/min in rabbits and more than 600 beats/min in mice 5. Respiratory monitors must be sensitive enough to detect small tidal volumes I Attaining surgical anesthesia A Toe, tail, and cloacal pinches B Most birds have a slow third-eyelid response; the loss of this response in birds indicates that anesthesia should be decreased C Response is not present in response to pinpricks to the cere II Anesthetic procedure recommendations (Tables 26-2 and 26-3) TABLE 26-3 A Preanesthetic agents can be used if needed B Inhalant anesthesia is preferred over injectable anesthesia 1. The safety and efficacy of injectable anesthetics vary among species and individual animals 2. It is difficult to titrate the dose of injectable anesthetics; inhalants are preferred 3. Injectable agents can be used for restraint for short diagnostic procedures, minor surgeries, or for induction to inhalant anesthesia D Use a nonrebreathing system for all birds weighing less than 7 kg 1. Restrain the bird by hand or use toweling 2. Place the bird’s head inside a clear plastic bag taped to the end of the Y- or T-piece while oxygen and inhalants are flowing or use a face mask 3. Hold the bird until it is relaxed and then maintain anesthesia with a mask or intubation E Endotracheal intubation is recommended for all birds 1. Birds weighing less than 100 g should be intubated for procedures longer than 30 minutes or for procedures involving the coelomic cavity; small face masks are commercially available or can be fashioned from 35- to 60-mL syringe cases and rubber gloves a. Very-small-gauge silicone endotracheal tubes sized 1.0 mm and 1.5 mm (internal diameter) are useful (noncuffed endotracheal tubes-silicone V-PAT-10, V-PAT-15; COOK Veterinary Products) 2. The glottis is easily visualized at the base of the tongue a. The trachea is composed of nonexpansible, complete tracheal rings; cuffs are not recommended b. Tissue swelling caused by tube-induced tracheitis can occlude the trachea in birds c. Small endotracheal tubes can be fashioned from catheters, the hub end of butterfly catheters, or red rubber feeding tubes; the hubs of these items fit into adapters made for commercially available 3.5- to 4.5-mm endotracheal tubes d. Making the tubes just long enough to ensure secure placement can minimize dead space e. Tubes should be taped securely to the bird’s beak to avoid the sensitive cere f. Tubes should be monitored for occlusion; mucous plugs and kinking are common; always have a replacement tube immediately available
Anesthetic Procedures in Exotic Animals
Overview
General Considerations
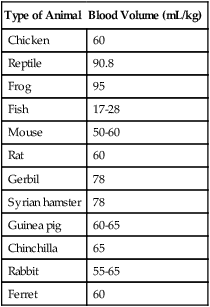
Type of Animal
Blood Volume (mL/kg)
Chicken
60
Reptile
90.8
Frog
95
Fish
17-28
Mouse
50-60
Rat
60
Gerbil
78
Syrian hamster
78
Guinea pig
60-65
Chinchilla
65
Rabbit
55-65
Ferret
60
Type of Animal
Packed Cell Volume (%)
Avian
<25
Reptile
<17 to 20
Amphibian
<20
Rodent/lagomorphs
<20
Mustelid
<25
Felid
<15
Swine
<20
Camelid
<20
Type of Animal
Packed Cell Volume (%)
Avian
>50 to 60
Reptile
>50 to 60
Amphibian
>60
Rodent/lagomorphs
>55 to 60
Mustelid
>60
Felid
>50
Swine
>50
Camelid
>50
Type of Animal
Glucose (mg/dL)
Avian
<150
Reptile
30-100
Amphibian
<50
Rodent/lagomorphs
<80-100
Mustelid
<70-100
Felid
<80
Swine
<60
Camelid
<60
Type of Animal
Time (hr)
Birds <100 g
0
Large psittacine bird
1 to 2
Raptor, ratite, fowl, waterfowl
12 to 24
Carnivorous reptile ingesting whole prey
>5 days
Reptile <200 g
2 to 4
Reptile 200 to 500 g
12
Reptile >500 g
24+
Amphibian
24
Fish
24
Rodent <200 g
0-2
Rodent >200 g
>6
Guinea pig
6-8
Ferret
3-6
Rabbit
0-24
Felid
24
Swine
24
Camelid
24 to 48
Species
Sites for Parenteral Access or Injection
Comment
Bird
IM, IO, IV: basilic, jugular, medial metatarsal
Rat and mouse
SQ, IM, IP, IO, IV: jugular, lateral tail
Lateral tail veins on mice are difficult to obtain blood from except by capillary action
Gerbil
SQ, IM, IP, IO, IV: lateral tail, saphenous, metatarsal
25-gauge needle
Hamster
SQ, IM, IP, IO (tibial crest), IV: lateral tarsus, cephalic, lingual, dorsal penile
IM: maximal volume 0.25 mL
IO: use 22-gauge spinal needle
IV: access is difficult, use 27- to 30-gauge needle
Chinchilla
SQ, IM, IP, IO, IV: femoral, cephalic, lateral saphenous, jugular, auricular, dorsal penile lateral abdomen, tail
IM: 23-gauge or smaller and maximum 0.3 mL
IV: 25-gauge or smaller needle
Guinea pig
SQ, IM, IP, IO (trochanteric fossa), IV: marginal ear, medial saphenous, lateral saphenous, proximal to hock, dorsal penile, jugular
Self-mutilation can occur with IM injections; vascular access is usually difficult with short, mobile, friable veins
Rabbit
SQ, IM, IP, IO (trochanteric fossa), IV: marginal ear, cephalic, lateral saphenous, jugular
Blood clots quickly, external jugular is primary drainage for head (swelling occurs if catheter remains in place)
Ferret
SQ, IM, IP, IO, IV: cephalic, jugular, lateral saphenous, lateral tail
Drug
Dose
Atropine
0.01-0.02 mg/kg IV, 0.02-0.08 IM
Glycopyrrolate
0.01-0.02 mg/kg IV, IM
Alphaxalone
10-14 mg/kg IV
Buprenorphine
0.01-0.10 mg/kg IM
Butorphanol
0.1-4.0 mg/kg IM
Codeine
30 mg/kg IM
Fentanyl
0.2 mg/kg IM
Morphine
0.1-3.0 mg/kg IV, 2.5-30 mg/kg IM, SQ
Diazepam
0.05-0.15 mg/kgIV, 0.2-0.5 mg/kg IM
Midazolam
0.05-0.15 mg/kg IV, 0.1-0.5 mg/kg IM
7.0 mg/kg intranasally
Detomidine
12 mg/kg intranasally
Ketamine >1 kg
15-20 mg/kg IM
<1 kg
30-40 mg/kg IM
Ketamine/midazolam
20-40 mg/kg IM+
4 mg/kg IM
Ketamine/xylazine
10-30 mg/kg IM+
2-6 mg/kg IM
Ketamine/midazolam/xylazine
40-50 mg/kg + 3.65 mg/kg +
10 mg/kg intranasally
Propofol
10 mg/kg slow IV infusion until effective
Up to 3 mg/kg increments for supplemental doses
Etomidate
10-20 mg/kg IM
Betamethasone
0.1 mg/kg IM
Dexamethasone
2-4 mg/kg IV, IM
Methylprednisolone
0.5-1.0 mg/kg (route?)
Prednisolone sodium succinate
0.5-1.0 mg/kg IV, IM
Ketoprofen
5-10 mg/kg IM, 2 SQ
Flunixin meglumine
1-10 mg/kg IV, IM
Phenylbutazone
3.5-7.0 mg/kg IV
Carprofen
2-10 mg/kg IM, 1 SQ
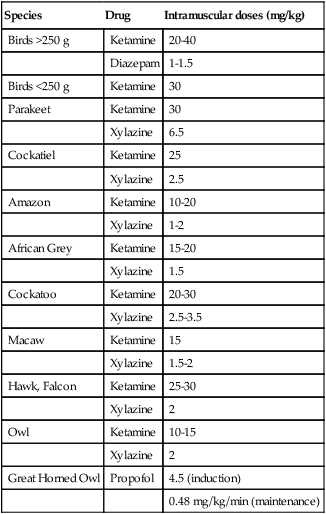
Avian Anesthesia (less than 15 kg)
Species
Drug
Intramuscular doses (mg/kg)
Birds >250 g
Ketamine
20-40
Diazepam
1-1.5
Birds <250 g
Ketamine
30
Parakeet
Ketamine
30
Xylazine
6.5
Cockatiel
Ketamine
25
Xylazine
2.5
Amazon
Ketamine
10-20
Xylazine
1-2
African Grey
Ketamine
15-20
Xylazine
1.5
Cockatoo
Ketamine
20-30
Xylazine
2.5-3.5
Macaw
Ketamine
15
Xylazine
1.5-2
Hawk, Falcon
Ketamine
25-30
Xylazine
2
Owl
Ketamine
10-15
Xylazine
2
Great Horned Owl
Propofol
4.5 (induction)
0.48 mg/kg/min (maintenance)
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


