Chapter 31 Anesthesia, Analgesia, and Sedation of Small Mammals
General Principles and Challenges in Anesthetizing Small Exotic Mammals
There are many challenges to be faced when one is anesthetizing very small patients. It is more difficult to obtain preoperative blood work, secure an airway or intravenous access, monitor anesthesia, and prevent hypothermia once the animal is anesthetized. Small mammals have higher metabolic rates and smaller glycogen reserves, predisposing them to hypoglycemia. Metabolism and excretion of parenterally administered drugs are faster; therefore they have shorter durations of action. Small mammals have higher oxygen consumption and may thus suffer irreversible central nervous system (CNS) injury more rapidly during respiratory arrest. Time becomes critical during anesthesia; complications occur faster, and the time for intervention is shorter. Much dosing information has been established in terminal studies of young, healthy laboratory animals, so critically evaluate the information prior to using it in a sick or debilitated pet animal. A recent large-scale prospective study reported an overall perianesthetic mortality rate of healthy pet rabbits and guinea pigs of approximately 1.39% and 3.80%, respectively, values 5 to 10 times higher than those found in dogs and cats.9 There is substantial room to decrease mortality during anesthesia in these patients.
Balanced Anesthesia/Analgesia
Isoflurane has been the most common anesthetic used in small exotic mammals for the last two decades and is often administered as the sole agent. Inhalant anesthetics have the advantage of allowing rapid changes in anesthetic depth and rapid recoveries owing to their low solubility in tissues and elimination via the respiratory tract. But inhalants also induce dose-dependent cardiopulmonary depression. Clinically, 1.3 to 1.5 times the minimum alveolar concentration (MAC) of an inhalant is required for immobility during surgery, but inhalants do not block activation of nociceptive pathways by noxious stimuli. High inhalant doses (1.5-3 times MAC) are required to block undesirable responses to pain, such as changes in heart rate, blood pressure, and respiratory rate, but the margin of safety at these doses is low and circulatory collapse can occur.121 While inhalants are still the main agents used for many procedures, there are many ways to minimize their use and thus decrease their adverse effects.
Preanesthetic Considerations
Patient Evaluation
A complete history should be obtained and a physical examination performed to assess preexisting clinical problems and record baseline values for comparison. Age should be considered, because the margin of safety is wider in younger animals. Obtain an accurate body weight for fluid and drug dose calculations. Evaluate hydration status, and correct dehydration prior to anesthesia. Compensatory mechanisms are blunted under anesthesia, exacerbating underlying hypotension and poor peripheral perfusion. Whenever possible, preanesthetic noninvasive blood pressure should be assessed to provide a more objective assessment of perfusion. Ideally, a complete blood count and biochemical profile should be performed prior to anesthesia. Severe anemia should be corrected, because the patient may not be able to compensate for decreased oxygen delivery. The packed cell volume (PCV) may decrease 3% to 5% during anesthesia because of vascular hemodynamic changes. In ferrets, isoflurane and sevoflurane are associated with significant reductions in PCV.77–79 Glucose concentration should be monitored and supplemented as necessary. The type of surgery to be performed and potential for blood loss should be assessed for fluid plan preparation.
Nutritional Status and Fasting
Prolonged anorexia leads to negative energy balance and hypoglycemia, requiring nutritional support in the perianesthetic period. Nutritional support for the debilitated patient is discussed in Chapter 38. Overnight fasting prior to anesthesia is not performed in small mammals because of their rapid gastrointestinal (GI) transit times and potential for GI stasis. Fasting has been advocated to reduce stomach and cecal volumes in large and obese rabbits, which can theoretically compress their small thoracic cavities, but this generally does not change GI volumes enough to be clinically important. Fasting exhausts glycogen stores, resulting in hypoglycemia, and may significantly contribute to perianesthetic ileus and potentially exacerbate pancreatic tumors in ferrets. Fasting reduces regurgitation in ferrets, but vomiting/regurgitation is uncommon in most other small mammal species. Rabbits and rats cannot vomit because of their well-developed cardiac sphincter and limiting ridge of the stomach, respectively. In general, recommended fasting times are 0 to 4 hours, with prudence used to weigh the risks and benefits in the specific patient. Schedule procedures early in the day to reduce fasting times. Many small rodents store food in their oral cavities; thus the mouth is cleaned out by gently syringing water into the oral cavity to remove food materials in tolerant awake patients or with wet cotton tips immediately after induction. Elevate the head during anesthesia if there is any concern of aspiration.
Equipment
Ventilators
Ventilators appropriate for small mammals must cope with the range of tidal volumes in the different species. In very small patients, this may be less than 1 mL; but some giant-breed rabbits might need to be as high as 500 mL. In general the three things that must be controlled by a ventilator are tidal volume, the rate at which the tidal volume is delivered, and the number of breaths per minute. The way in which each of these is controlled varies from ventilator to ventilator.45
Clinical Techniques
Vascular Access
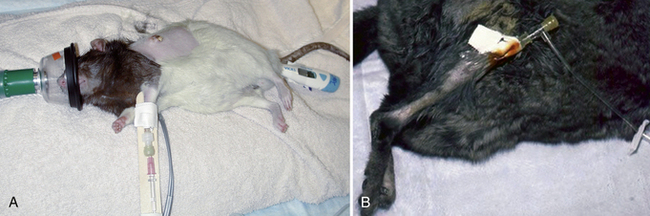
Vascular access is necessary for replacement fluids and to deliver anesthesia and emergency medications. Sites for intravenous catheterization include the cephalic, lateral saphenous, and femoral veins in larger mammals and the superficial lateral tail veins in the rat (Fig. 31-1). Sedation may be necessary for intravenous catheter placement. A surgical cut-down procedure under anesthesia is generally necessary for jugular catheterization. Small-bore over-the-needle catheters (≤24 gauge) are used. The catheter site should be aseptically prepared; catheters are then secured with tape and/or sutured in place and bandaged for extra security. Jugular catheters, if left indwelling, require 24-hour monitoring, as fatal hemorrhage can occur if the patient pulls or chews on the catheter and damages the vessel. Many small rodents, even when severely compromised, are intolerant of bandaging material and indwelling catheters and will attempt to remove them.
Intraosseous (IO) cannulation can be useful in smaller patients or during cardiovascular collapse (see Fig. 31-1).89 Products that can be used as IO cannulas include 18- to 24-gauge 1- to 1½-in. spinal needles or 18- to 25-gauge 1-in. hypodermic needles, depending upon the animal’s size. The cannula should be long enough to extend through one-third to one-half the length of the medullary cavity. A wire stylet reduces the potential for a bone core. Prepare several hypodermic needles (25- to 18-gauge) with wire stylets (stainless steel suture) and sterilize them for IO catheterization. Common sites for IO cannula placement include the trochanteric fossa of the femur and the tibial crest (see Fig. 31-1, B). Placement is similar to that of a normograde intramedullary pin and requires strict aseptic technique during placement and maintenance. Once the cortex is penetrated, the cannula should advance easily, with little resistance. Flush the cannula with heparinized saline immediately to prevent clotting. Cover the insertion site with an antibiotic ointment and secure the cannula with tape, suture, and a bandage. IO cannulas can remain patent for 72 hours without flushing but should be flushed with heparinized saline twice daily if fluid therapy is not continuous. Complications associated with IO catheterization include penetration of both cortices, failure to properly enter the medullary cavity, and extravasation of fluids with associated pain.89 IO catheters are used primarily for short-term vascular volume expansion until an IV catheter site can be obtained. Many animals become uncomfortable on limbs supporting IO catheters even after short-term placement. IO catheterization is contraindicated in septic patients and those with metabolic bone disease. Osteomyelitis may occur owing to duration or placement of the IO catheter; administration of alkaline or hypertonic solutions can contribute to osteomyelitis and cause pain.89 Dilute these solutions before administration and flush the catheter with heparinized saline after any drug injection.
Intubation
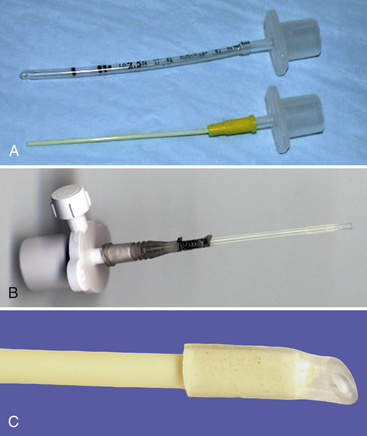
Specialized endotracheal tubes and light sources are available to aid intubation of small mammals. The smallest commercial uncuffed tubes have an internal diameter (ID) of 1 mm. However, tubes of less than 2-mm ID are often highly flexible and kink easily. The smallest-diameter cuffed tube is 3-mm ID. Uncuffed tubes do not provide a sealed airway, so clean the oral cavity prior to intubation, elevate the head, and monitor during the procedure. If you are using a cuffed tube, the cuff is carefully inflated with just enough air to prevent leakage when 10 to 15-cm H2O pressure is applied. Very small mammals may be intubated with Teflon IV, red rubber, or urinary catheters (Fig. 31-2, A). Care is taken to ensure that no sharp edges are present at the end. This is achieved by using a small piece of silicone tubing over the end of the catheter (see Fig. 31-2, B,C).
Endotracheal tube obstruction is detected by monitoring for a prolonged expiratory phase. Anticholinergics reduce the production of mucus and mucous plug formation but also increase mucous viscosity, making it harder to clear secretions. The use of an endotracheal tube with a Murphy eye decreases the likelihood of mucous occlusion. Humidification of the gases reduces mucous plug formation. Commercial endotracheal tube humidifiers are available (Humidi-vent Mini Agibeck Product, Hudson RCI, Temecula, CA). Disadvantages of their use include increased dead space and plugging of the filter with secretions.
Care must be taken to minimize head and neck movement in intubated patients, as movement of the tube and changes in positioning may induce mucosal trauma. Sublaryngeal tracheal injury, ulceration, and postintubation tracheal strictures have been described in rabbits from several institutions that were intubated with both cuffed and uncuffed endotracheal tubes.38,92 Other factors that predispose to mucosal injury include ventilation technique and endotracheal tube disinfection protocols.
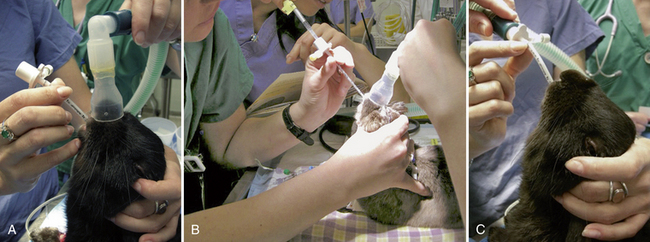
Ferret intubation is straightforward and should be performed routinely as in the cat. All pet rabbits over 1-kg body weight can be intubated, but this is more difficult. Rabbits have large incisors, long narrow oral cavities, and thick tongues, making laryngeal visualization more difficult, and laryngospasm is easily induced. There are a number of tracheal intubation techniques that have been advocated for use in rabbits: direct visualization of the larynx with a laryngoscope and intubation, blind intubation with the neck in extension, endoscope-guided intubation, and nasotracheal intubation. Apply topical local anesthetic on the larynx 60 seconds before intubation to decrease laryngospasm. The authors use the blind technique routinely. Guide the tube to the larynx and listen for louder breath sounds, place one drop of 2% lidocaine via tomcat catheter through the endotracheal tube directly on to the larynx, then use the same approach for intubation (Fig. 31-3). Placement of the tube is confirmed by visualizing the movement of water vapor in the tube, by the rabbit coughing, by auscultation of breath sounds in the lungs, and definitively by attaching a capnograph and seeing a characteristic capnographic trace. Rabbits are obligate nasal breathers and the patency of both nares must be carefully assessed postextubation, especially if the animal has been in dorsal recumbency. Reported complications include postextubation obstruction, respiratory arrest, and tracheal mucosal injury.38,92 Intranasal intubation or catheterization can be used in the rabbit if endotracheal intubation cannot be performed or for complicated oral procedures, such as extensive dental procedures. The ventral meatus of the rabbit’s nasal passage is surprisingly large. A blind intubation technique with a 2.5- to 3-mm ID endotracheal tube is used, but these are often stiff for smaller rabbits; standard IV tubing has a thinner wall and so is more flexible. IV tubing can be cut to the appropriate length and a bevel fashioned on the distal end; the proximal end can be fitted with a 2- to 2.5-mm endotracheal tube adapter. Administer anticholinergics with nasal intubation, as vagally mediated bradycardia can be associated with this procedure. The tube is passed through the ventral meatus with the head held in a normal or slightly extended position. Because of the difficulties of intubating rabbits, newer techniques such as laryngeal mask airways are being evaluated.7,66,111
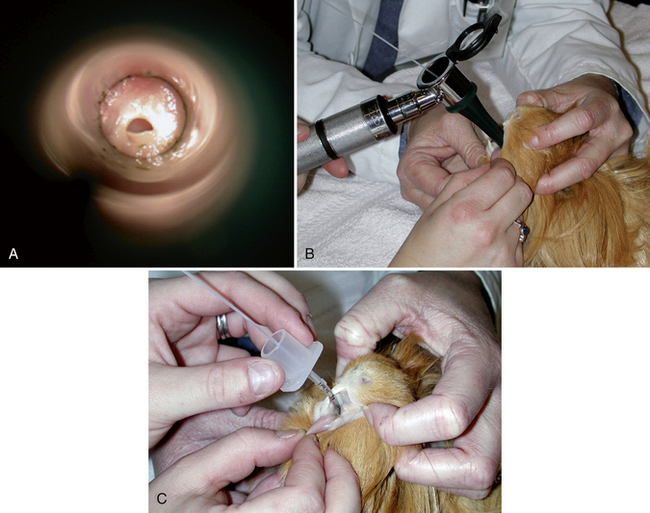
Rodents also have a long narrow oral cavity, but equipment and techniques for intubating small rodents have been developed.87,117 In guinea pigs and chinchillas, orotracheal intubation is complicated by the fusion of the soft palate to the base of the tongue, creating the palatal ostium, which is highly vascular and easily traumatized (Fig. 31-4, A). Endotracheal tubes of 1.0- to 2.5-mm ID are most often needed for small rodents. Very small rodents may be intubated with catheters or IV tubing as in the rabbit, but occlusion with mucous plugs, due to the small internal diameter of these tubes, occurs frequently. An otoscopic cone, modified pediatric blade, commercially available rodent work stands and intubation packs, or endoscopy can help facilitate intubation. Otoscopic cones that have been modified by removing a section laterally can facilitate visualization of the epiglottis and direct placement of the endotracheal tube. A stylet placed first may help facilitate endotracheal tube placement (see Fig. 31-4, B,C). Endoscopy provides the best visualization of the epiglottis and minimizes trauma during tube placement. Nasal intubation/catheterization has also been performed for guinea pigs and other small rodents with similar issues arising as for rabbits.
Preanesthetic Medications
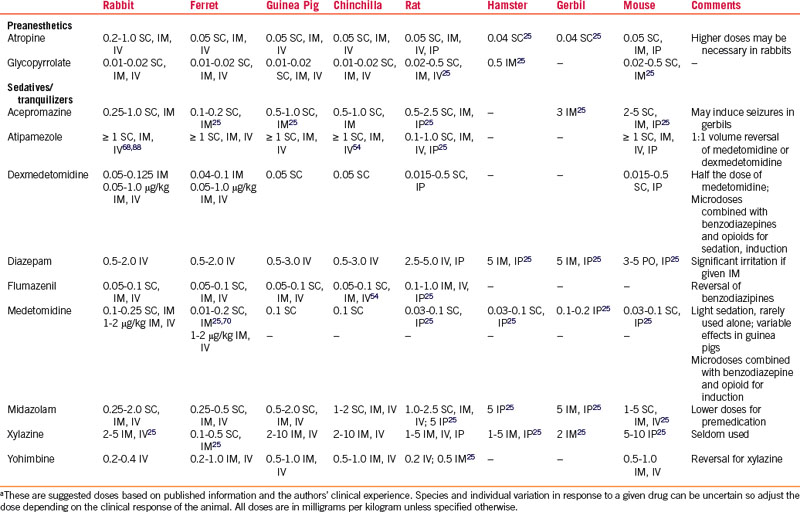
Parasympatholytics are most commonly used to minimize salivary and bronchial secretions and vagally induced bradyarrhythmias, but they can increase secretion viscosity. Many rabbits have high circulating concentrations of atropine esterases, thus reducing the efficacy of atropine and prompting the use of much higher atropine doses, with redosing as often as every 10 to 15 minutes during bradycardia.44 Large doses of parasympatholytics can alter GI motility in hind-gut fermenters; therefore the lowest doses necessary are used.48 Glycopyrrolate has a longer duration of activity than atropine (Table 31-1). Glycopyrrolate increased heart rates in rats for 240 minutes, versus 30 minutes with atropine.86 The same study demonstrated that 0.1 mg/kg glycopyrrolate administered to rabbits increased heart rate for an equal duration to the rats, but atropine doses ≤2 mg/kg did not increase heart rate for any duration.86
Injectable Medications
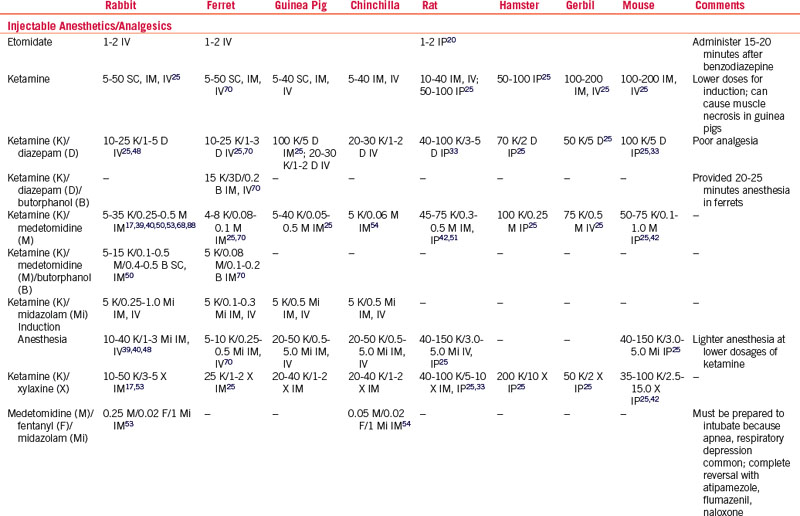
A considerable increase in the use of injectable anesthetic combinations in small exotic mammals has recently been documented.* Certain breeds or even strains of small mammals have differing responses to injectable anesthetics.5 There is little published work evaluating different injectable combinations for common surgical procedures in pet animals of differing age, sex, breed, and health status in the clinical setting,40,88 and so use caution when extrapolating injectable doses from laboratory animal studies. Using reversible injectables provides better control of anesthetic depth, less hypothermia, less cardiopulmonary depression, and shorter recovery times.54–5698
Sedatives and Tranquilizers
Acepromazine
In ferrets, acepromazine provides adequate sedation for simple procedures such as ear cleaning,71 however, one author discourages its use in ferrets owing to its vasodilatory effects (see Table 31-1).64 Acepromazine has been used extensively in rabbits and rodents and reported doses vary widely.4,48,120,122 The peak effect in rabbits is often not seen for 30 to 40 minutes, even when given IV. It is used in laboratory rabbits specifically for blood collection because of the drug’s vasodilatory effects. Vasodilation occurs at very low doses of acepromazine, therefore low doses will not avoid this effect. Animals that are hypovolemic, anemic, or hypotensive before anesthesia should not receive acepromazine. Acepromazine has also been shown to decrease tear production in rabbits.35 Acepromazine is not recommended for use in gerbils because it may lower the seizure threshold.49
Benzodiazepines
Diazepam can be administered PO or IV, but if used IV, the patient must be monitored for hypotension caused by the propylene glycol found in many diazepam formulations (see Table 31-1). IM and SC absorption is erratic because of the solution’s high osmolality, which is due to an increased pH with this carrier. Because parenteral formulations of diazepam are available, large volumes must often be given. Transient lameness after IM injection has been reported in ferrets.72,74 Diazepam can be used alone in rabbits to provide preoperative anxiolysis and sedation,15 but it is most commonly used in combination with other drugs to enhance their activity or decrease muscle rigidity.
Midazolam is a short-acting benzodiazepine that is water-soluble, so it can be given IM (see Table 31-1). Midazolam has been used as a sole sedative for minor, nonpainful procedures in rabbits and rodents. Lower doses are most often used for preanesthetic sedation. Flumazenil will reverse midazolam, but because its half31-life is shorter than that of midazolam, resedation may occur. Titrate the flumazenil dose to avoid the reversal of the beneficial anxiolysis, sedation, and muscle relaxation. Benzodiazepines coupled with opioid medications provide sedation and preoperative analgesia.
Alpha-2 Agonists
Xylazine, medetomidine, and now dexmedetomidine are the most commonly used alpha-2 adrenergic receptor agonists in small exotic mammals (see Table 31-1). Their major advantages are that they provide good muscle relaxation and can be reversed with yohimbine, atipamezole, or tolazoline (see Table 31-1). If all analgesic components are reversed, postoperative analgesia should be provided before reversal of anesthesia. These drugs can have significant cardiopulmonary effects, including respiratory depression, second-degree heart block, bradyarrhythmias, and increased sensitivity to catecholamine-induced cardiac arrhythmias (xylazine).53,54 Because of the many adverse effects reported for this class of drugs, their use is not recommended in small exotic mammal patients with evidence of cardiopulmonary compromise. Supplemental oxygen and assisted ventilation should always be available when these drugs are being used.
Xylazine/ketamine given over multiple anesthetic episodes and detomidine alone or in combination with ketamine or diazepam have been associated with myocardial necrosis and fibrosis in rabbits.61,80 Significant breed and strain differences in response to medetomidine sedation doses have been reported for rabbits and rats.5,6,48 In rabbits, ketamine/medetomidine allowed for more rapid intubation, a greater isoflurane-sparing effect, and less esophageal temperature loss than with ketamine/midazolam, but the rabbits thus treated were more prone to laryngospasm.40 Medetomidine/ketamine provided better quality and duration of surgical anesthesia (38.7 ± 30.0 minutes) in healthy rabbits than medetomidine/midazolam/fentanyl (MMF). Arterial pH and PaO2 were significantly decreased in both groups and apnea occurred postintubation with MMF.53 Atipamezole (10%-20% of calculated dose) may reduce these adverse effects without reversing their anesthetic and analgesic effects, but supplemental oxygen must be available.
The effects of alpha-2 agonists with ketamine seem to be less uniform in guinea pigs, and they may not become sufficiently anesthetized.96 Chinchillas administered MMF IM had well-controlled anesthesia for 1.5 hours, but the heart rate (HR) and respiratory rate (RR) were decreased and recoveries were prolonged without reversal.54 Chinchillas receiving medetomidine/ketamine had greater HR and RR depression, and recovery was longer than with MMF combinations (Table 31-2).54 Alpha-2 agonists are combined with ketamine for IP injection for surgical anesthesia with good muscle relaxation in rats and mice.4,96
Induction and Maintenance of Anesthesia
Preoxygenation should be performed routinely, and always when there is potential for hypoxemia. Preoxygenation can produce an oxygen reservoir and the benefits are achieved in ≤1 minute of high inspired oxygen concentration in the healthy patient but may take ≥5 minutes in a patient with compromised respiratory function. Preoxygenation is accomplished with an oxygen cage or an induction chamber in the unrestrained animal or with a face mask in the physically restrained patient. Levels rise more slowly in an unprimed oxygen cage or induction chamber. It is expected that PaO2 will decrease rapidly if oxygen delivery is interrupted and the animal begins to breathe room air again. Supplemental O2 should be supplied throughout the anesthesia, and intubation should be performed whenever possible.
Injectable Anesthetics
Ketamine
Ketamine is a noncompetitive n-methyl-d-aspartate (NMDA) receptor antagonist that can prevent central sensitization, provide analgesia, and cause dissociative anesthesia (see Table 31-2). Ketamine is generally well tolerated in the stable patient but it has sympathomimetic properties that can cause increases in HR, myocardial contractility, and peripheral vascular resistance. It is a negative inotrope; therefore patients that are highly stressed (especially rabbits and chinchillas) may become hypotensive following ketamine injection because they have maximized their sympathetic output, thus “unmasking” the negative inotropic effect of the drug. Patients with hypertrophic cardiomyopathy may be at an increased risk of cardiac failure, as they may not be able to cope with the increased myocardial demand caused by sympathetic stimulation. Renal impairment can markedly prolong recovery. The authors do not recommend using ketamine for patients with cardiac or renal disease or in severely stressed small mammal patients. Salivation is increased and secretions can obstruct the airway. In small rodents, higher IM doses have been associated with muscle necrosis at the injection site. Ketamine has a very short duration of clinical effect in rabbits and varies within strains of rabbits, partly owing to redistribution and renal elimination.5 Another disadvantage is the potential for the development of apneustic ventilation, a pattern characterized by a prolonged pause after inspiration. Despite these disadvantages, ketamine combined with one or more drugs is still considered a first-choice injectable anesthetic.97
Ketamine is commonly combined with alpha-2 agonists or benzodiazepines as induction agents to improve relaxation and anesthetic depth (see Table 31-2).* Ketamine/alpha-2 agonist combinations, particularly at higher doses, produce mild-to-severe dose-dependent hypotension and bradyarrhythmias; ketamine/benzodiazepine combinations produce less cardiopulmonary depression and analgesia but provide good muscle relaxation.40 Low-dose ketamine/midazolam IV is used by the authors to induce anesthesia in rabbits, ferrets, and small rodents; it is given IP in very small rodents. Opioids added to ketamine combinations further reduce the dose and minimize the adverse effects of each drug. Reversal of other drugs in ketamine combinations allows for its specific undesirable effects to become apparent.
Tiletamine-Zolazepam
In mice, tiletamine-zolazepam (Telazol, Fort Dodge, IA) alone appears to produce poor analgesia, but in combination with xylazine (7.5 mg/kg tiletamine-zolazepam and 45 mg/kg xylazine) this improved, with an anesthetic time of 30 to 90 minutes (see Table 31-2). In rats, doses of 30 to 60 mg/kg were tested and found to have a dose-dependent effect on the duration of anesthesia (59-124 minutes), but not much change in cardiorespiratory effects was seen over this high dose range.104 Much smaller doses of tiletamine-zolazepam (1.5-3.0 mg/kg) are used in ferrets with xylazine (1.5-3.0 mg/kg) or with xylazine (1.5 mg/kg)/butorphanol (0.2 mg/kg) with a dose-dependent duration of effect.70 Tiletamine-zolazepam has been reported to cause nephrotoxicity, related to the tiletamine, in New Zealand white rabbits, and this appears to be dose-dependent.18 The advantage of this combination over ketamine is that much smaller volumes of drug are needed, so it is less likely to cause muscle damage if given IM.
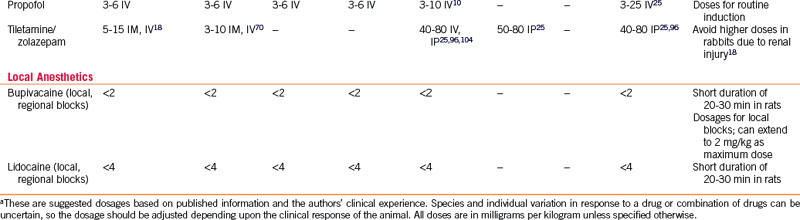
Propofol
The need for IV access and ventilatory support limits the use of propofol. Rapid, excitement-free induction and recovery occur at clinically relevant doses in most mammals (see Table 31-2). Early studies evaluating propofol anesthetic maintenance as the sole anesthetic agent in rabbits demonstrated a very narrow therapeutic window,2 but balanced anesthesia may produce propofol-sparing effects, thus reducing its respiratory disadvantages.73,81 The authors suggest a calculated propofol induction dose be given in one-quarter increments, each administered over 30 to 60 seconds, to allow more accurate dosing and minimize apnea and hypotension during induction. The effects of propofol given IP in small rodents are unpredictable; very little information is available on using this drug via this route in small exotic mammals.83 New formulations of propofol have been developed that may change some of the uses in these species. A clear solution of nanoparticulate propofol in an aqueous base eliminates the addition of other solvents and gives this agent a much longer shelf life after the container is opened.95
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree