Mandy J. Meindel, Melinda J. Wilkerson
Anemia
Anemia, defined as low erythrocyte or hemoglobin concentration, or low hematocrit, is a common pathologic state in horses. General causes of anemia include blood loss or hemorrhage, hemolysis, and inadequate erythropoiesis. Clinical signs are a reflection of inadequate oxygenation of tissues and are determined by severity, rate of development, and the physical demands of the horse. Tachycardia, tachypnea, reduced exercise tolerance, lethargy, and pale or icteric mucous membranes are common findings with anemia. Signs of chronic anemia in horses are often subclinical until the hematocrit is less than 15%. In acute-onset anemia (occurring within 12 to 24 hours), clinical signs may be more severe and include colic, laminitis, blindness, ataxia, and collapse secondary to oxygen deprivation or hypovolemia.
Evaluation of anemia requires interpretation of hematocrit, erythrocyte concentration, and erythrocyte indices (mean corpuscular volume [MCV], mean corpuscular hemoglobin [MCH], and mean corpuscular hemoglobin concentration [MCHC]) and careful examination of a blood film and, in some cases, examination of bone marrow. Horses are unique in their physiologic response to anemia in that they generally do not have overt evidence of regeneration (release of reticulocytes) in the peripheral blood, so other findings must be assessed to evaluate the regenerative response (e.g., anisocytosis with macrocytosis seen during blood film evaluation or by an increased MCV). Reticulocytes may be released in significant numbers in severely anemic horses or horses given high doses of exogenous erythropoietin. Newer, automated hematology analyzers1 can detect circulating reticulocytes using an RNA-sensitive dye (oxazine). Microcytosis detected in cases of iron deficiency anemia is most consistent with chronic external blood loss. Low MCHC and MCH also support iron deficiency. Nonregenerative anemia of greater than 1 week’s duration is supported by erythrocyte indices in the normal reference interval for healthy horses.
Evaluation of a blood film should always be performed to aid in classification of the anemia and possibly to identify an etiologic agent. Erythrocyte morphologic features associated with regeneration, such as nucleated erythrocytes, basophilic stippling, or polychromasia, are rarely seen in horses. Eccentrocytes, Heinz bodies, and pyknocytes may be observed in cases of oxidative damage, for example, with red maple leaf toxicosis and erythrocyte enzyme or cofactor deficiencies. Erythrocytes should be examined for hemoparasites, including Babesia and Theileria spp. Agglutination on a blood film suggests the presence of erythrocyte surface immunoglobulins and an immune-mediated etiology. However, agglutinated erythrocytes must be confirmed and differentiated from rouleaux, a normal finding in horses, by mixing 9 drops of 0.9% saline solution and 1 drop of anticoagulated whole blood and examining a wet mount preparation. This saline dilution procedure will disperse rouleaux but not agglutination.
An absence of abnormal peripheral blood findings can pose a diagnostic challenge and may warrant a bone marrow aspirate to assess for a regenerative response and look for an underlying etiology. Regenerative response in the bone marrow is indicated by an increase in erythroid precursors, with evidence of increased polychromasia (assuming the marrow is not diluted with peripheral blood) and a decreased myeloid-to-erythroid ratio secondary to erythroid hyperplasia. In general, hemolytic anemia is regenerative within a few days of onset. External blood loss anemia, as with vascular lacerations or gastrointestinal blood loss, is initially regenerative but may become nonregenerative over time because iron necessary for erythropoiesis is depleted. Quantities of Prussian-blue–stained iron seen in bone marrow during iron deficiency anemia are low. In contrast, following internal blood loss (e.g., uterine artery or splenic rupture), iron is recycled for erythropoiesis because erythrocytes are autotransfused back into circulation from the lymphatics. Inadequate erythropoiesis is supported by decreased numbers of erythroid precursors or a maturation arrest at a stage of erythroid maturation and an increased myeloid-to-erythroid ratio.
Regenerative Anemia
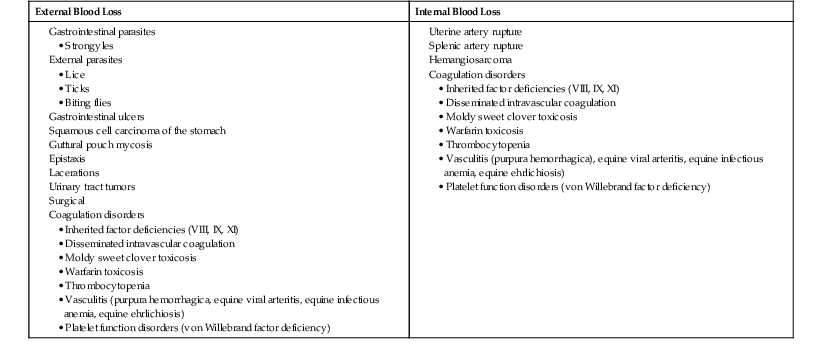
Blood Loss
Anemia caused by blood loss can result in a variety of clinical and laboratory findings, depending on the severity, chronicity, and origin of blood loss. Causes of blood-loss anemia are listed in Box 112-1. External blood loss may be obvious, as in cases of lacerations, or may be more subtle, as with gastrointestinal parasitism. Internal blood loss may be harder to diagnose unless a relevant clinical history is available. In those situations, anemia is often severe and may be accompanied by overt clinical signs of hypovolemic shock.
Anemia will not be immediately apparent until fluid shifts into the vasculature and dilutes the remaining erythrocytes; this fluid shift occurs within 12 to 24 hours. A decrease in total protein, albumin, and globulin concentrations is also expected in the face of concurrent loss of protein-rich plasma and dilution of blood constituents as fluid shifts into the vasculature. Hypoproteinemia and anemia generally resolve quicker with internal hemorrhage than with external hemorrhage because proteins and most of the erythrocytes are returned to the plasma through the lymphatics. With external blood loss, erythrocytes and plasma proteins are lost, which leads to compensation by the bone marrow and liver to replenish the circulating pool of cells and proteins. In chronic external blood loss of sufficient severity, iron depletion leads to a nonregenerative anemia secondary to ineffective erythropoiesis.
Immune-Mediated Hemolytic Anemia
Immune-mediated hemolytic anemia (IMHA) is a type II hypersensitivity reaction in which antibody targets erythrocytes for macrophage destruction (extravascular hemolysis) in the spleen or by complement-mediated lysis of antibody-coated cells in the vasculature (intravascular hemolysis). Immune-mediated hemolytic anemia is classified as either primary or secondary. In primary IMHA, antibodies are formed against normal erythrocyte epitopes (i.e., autoimmune hemolytic anemia) resulting from a loss of self-tolerance to erythrocyte antigens. Autoimmune IMHA is rare in horses and difficult to confirm. In alloimmune hemolytic anemia (another form of primary IMHA), seen with incompatible blood transfusions and neonatal isoerythrolysis, antibodies are produced against foreign erythrocyte antigens. Neonatal isoerythrolysis is an important cause of life-threatening IMHA in newborn foals and mules, caused by maternal alloantibodies directed against antigens on the foal’s erythrocytes that are inherited from the sire and not expressed by the mare. Sensitization of the mare’s immune system develops after leakage of foal erythrocytes into the maternal circulation during parturition and becomes clinically significant after the birth of subsequent foals that express the sire’s antigens. Extravascular or intravascular hemolysis occurs after the newborn foal ingests maternal immunoglobulin G (IgG) alloantibodies in the colostrum. Of the 32 blood group antigens on equine erythrocytes, alloantibodies have been reported most commonly for Aa, Qa, Ab, Ac, Db, Pa, Pb, Qc, and Ua. Treatment includes muzzling the foal to prevent further exposure to maternal antibody, transfusion, and supportive therapy.
Secondary IMHA occurs when antibodies are formed against foreign epitopes that coat erythrocytes or against neoantigens that develop as a result of an underlying condition, including infection, neoplasia, or drug exposure. Immune-mediated hemolytic anemia associated with clostridial infections is common among infected horses. It has been proposed that clostridial toxins damage the erythrocyte membrane, exposing new or altered epitopes that stimulate antibody production. Other causes include equine infectious anemia (EIA) virus; streptococcus; lymphosarcoma; melanoma; drugs or toxins, including penicillin, sulfonamides, and organophosphates; and other systemic diseases, such as protein-losing enteropathy and purpura hemorrhagica. In EIA viral infection, the hemagglutinin subunit of EIA virus binds erythrocytes, causing antibody to be formed against the altered erythrocytes that then leads to binding and activation of the complement cascade and hemolysis. Drugs that induce antibodies act as haptens and are only immunogenic when conjugated with a large carrier molecule, like albumin, in the blood or an erythrocyte membrane protein. Such complexes induce antibodies to either the drug alone, to an antigen that is part drug and part carrier protein, or to the carrier protein alone. Penicillin G, a drug commonly used in horses, causes IMHA in some horses. When exposure to the drug is known, cessation of the drug is the best approach to therapy.
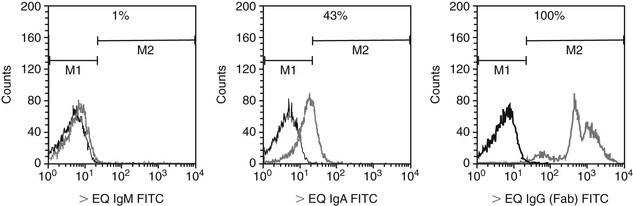
Clinical signs of both primary and secondary IMHA may include fever, depression, icterus, and hemoglobinuria. Diagnosis of IMHA is supported by detection of severe regenerative anemia (packed cell volume [PCV] is typically less than 20%, and MCV is increased), and erythrocyte agglutination may be seen on the blood smear. Confirmation of an immune-mediated cause requires the detection of antibodies on the surface of erythrocytes with a direct antiglobulin (Coombs’) test or direct immunofluorescence test using flow cytometry. Both tests incubate a patient’s washed erythrocytes with equine-specific antiserum. The flow cytometry assay uses fluorescently tagged antiequine antibodies and detects a lower concentration of cells coated with antibody than Coombs’ test because the flow cytometry assay is not dependent on agglutination as an endpoint. In addition, the flow cytometry assay identifies the proportion of cells bound with class-specific antibodies (IgG, IgM, or IgA) and is useful in monitoring the patient during the course of treatment. A recent modification of an indirect agglutination-based test for penicillin-induced hemolytic anemia developed by Kansas State University’s Clinical Immunology Laboratory consists of coating donor horse erythrocytes with penicillin G. Drug-coated cells are incubated with the patient’s serum and then incubated with fluorescently tagged antiequine IgG, IgM, and IgA antibodies. An example of a positive test for serum IgG and IgA antibodies reactive against penicillin-coated cells in a horse with penicillin-induced hemolytic anemia is depicted (Figure 112-1).
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree