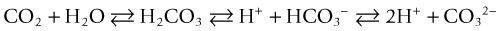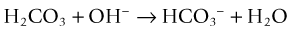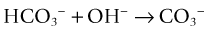Chapter 25 Advanced Water Quality Evaluation for Zoo Veterinarians
As indicated in the basic water quality chapter (see Chapter 23), conditioning water is much more involved than monitoring and adjusting water for the basic parameters such as temperature, pH, dissolved oxygen (DO), ammonia, nitrite, nitrate, pH, salinity (conductivity), hardness, and alkalinity. This chapter is designed to inform veterinarians, many of whom oversee the water quality laboratories in zoos and aquariums, about the more complex aspects of water conditioning. These may include oxidation-reduction potential, total organics, metals, microbial dynamics, and organic contaminants. Most of the discussion will focus on marine systems because of their complexity, but variations with freshwater will be highlighted. This chapter is intended to be a primer; for more detailed information, see Spotte24 and Clesceri and colleagues.5
Advanced Water Quality
Dissolved Gases
Partial pressures are extremely important but are underanalyzed in an aquarium setting unless direct gas bubbles are forming on the glass of the aquarium or emboli are observed in the fish. Chronic and/or sublethal levels may cause morbidity and mortality from secondary factors. Partial pressures are measured using a gas tensionometer or saturometer. Baseline pressures are dependent on temperature and salinity and should be analyzed with methods provided in the appropriate literature.8 Because of its insolubility and biologic unavailability, nitrogen is usually the culprit, but oxygen and carbon dioxide may cause issues in extreme cases. If chronic dissolved gas levels cannot be resolved, fish should be encouraged to swim below the compensation depth, which would prevent bubble formation. This may be accomplished by feeding and shelter strategies that keep them at depth.
Carbon Dioxide
Nitrogen is the most abundant gas in aquatic systems, but oxygen and CO2 concentrations are the most dynamic because of manipulation by biologic activity. In an ecologically balanced system, oxygen and CO2 tend to be inversely related in terms of their activity because one is constantly being exchanged for the other between plants or photosynthetic protoctists (e.g., algae, cyanobacteria) and animals, which has resulted in many ocean symbioses; thus, this effectively using light energy to drive chemical equilibria and stability in the system.1 Probably the most important part that CO2 plays in the aquarium setting is its role in the carbonate alkalinity system, which controls the pH level and its stability; this system is crucial to maintaining organisms’ health. Other factors affecting this system include carbonic acid, carbonate, bicarbonate, and hydrogen ion levels. This is especially true in marine environments, in which animals have adapted to a very stable pH and narrow pH range.
CO2 reacts with water to form carbonic acid (H2CO3), which dissociates to bicarbonate (HCO3−) and then carbonate (CO32−) and hydrogen ions (H+), chemically proportioned by temperature, pressure, and salinity equilibria24:
It also converts bicarbonate into carbonate13:
Denitrification
In cases in which water changes are not feasible or at lower levels than desired, denitrification is the process of turning nitrate into nitrogen gas through anaerobic microbial action. An additional carbon source is needed through the use of methanol or sulfur, which helps facilitate the autotrophic denitrification process in which the carbon issued is from the CO2. These are complex processes and denitrification should not be attempted without experienced staff because toxic elements may be produced and released into the main water system if the procedure is not performed correctly. It is important to note that our understanding of bacteria in the nitrogen cycle is in its infancy; once more information is gained, we could significantly alter our water-conditioning capabilities. For example, as recently as 2002, it was discovered that bacteria could perform anaerobic ammonium oxidation (anammox) to N2 gas. These four genera of anammox bacteria were identified as Brocadia, Kuenenia, Scalindula, and Anammoxoblobus and are responsible for 24% to 67% of nitrogen loss in marine systems.9 They may be grown autotrophically with CO2 as the only carbon source, thus eliminating the complex dependency on several bacterial pathways, including anoxic denitrification using methanol or sulfur. It is currently being applied in industrial use and is highly experimental but could be used in zoo and aquarium settings. We would then not have to depend on complex biologic filtration systems, which are prone to failures.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree