Chapter 10. Advanced Imaging Modalities
SECTION A Positron Emission Tomography (PET)
Amy K. LeBlanc
KEY POINTS
• Positron emission tomography (PET) and fused PET/computed tomography (CT) are widely used as non-invasive imaging techniques for staging and management of human cancer.
• 18 FDG is the most common PET tracer used in clinical practice, which can readily identify areas of increased glucose utilization within the body, such as malignancy and inflammation.
• PET will be used for staging and management of veterinary patients with cancer as access to necessary equipment and radiopharmaceuticals improves, representing an important step forward in the practice of veterinary oncology.
POSITRON EMISSION TOMOGRAPHY
Positron emission tomography (PET) is a nuclear medicine technique that uses a radiopharmaceutical tagged with a positron-emitting isotope to map distribution of radioactivity throughout the body. 1 Distribution of the radiopharmaceutical is dependent on specific cellular function(s), providing an assessment of physiologic and/or metabolic processes in the body. Recent advances in scanner design and performance, in addition to PET/CT fusion, have moved PET to the forefront of oncologic imaging. PET technology was first used in the 1980s for study of neurologic and cardiac diseases through mapping of glucose metabolism with a commonly used positron-emitting tracer, 2-[ 18 F]-fluoro-2-deoxyglucose ( 18 FDG). 2 PET is now widely used for the staging and management of human cancer patients, based on the increased glucose transport and metabolism in tumors compared with surrounding normal tissues. 3,4
Physics of Positron Emission and Detection
The images produced with PET use unique physical properties of positron-emitting radionuclides, which are “neutron-deficient” unstable isotopes that decay by emission of positrons (e + ), or positively charged electrons with mass equivalent to an electron. 5 The positron emitted from the nucleus loses energy through collision with electrons in the surrounding tissue until it annihilates with an electron from an adjacent atom. The energy produced by the annihilation reaction is in the form of two 511 keV photons that are emitted about 180 degrees apart. 5,6 Detection of these two photons, and not the positron itself, is the foundation of PET imaging.
PET Scanners
The PET camera is designed to detect the pair of annihilation photons from the decay of the positron-emitting isotope and is comprised of a ring of block detectors that encircles the patient. The two annihilation photons produced by positron emission are captured in coincidence by opposing detectors around the patient, meaning that detectors record “true” coincidence events. 6 A true coincidence is defined by a pair of unscattered photons arising from a single annihilation that arrive within the coincidence time window. 5 Detection of the photons by opposing detectors means that the annihilation reaction, and thus the location of the radiopharmaceutical, occurred somewhere along a line between the detectors, which is referred to as the line of response (LOR). These paired events are stored in matrices or sinograms where each row in the matrix represents a projection of the activity distribution in the patient at a specific angle and axial position. 6 An image reconstruction algorithm is applied to recover the radioactivity distribution, thus using LORs to indirectly map the functional process that created the distribution of positron emitter. The resulting images represent radiopharmaceutical accumulation in specific areas of the body, representative of the underlying biologic process of interest ( Figure 10-1 ).
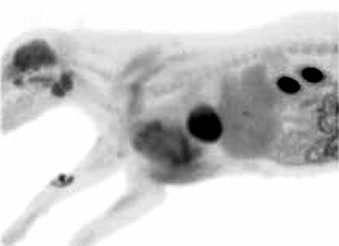 |
| FIGURE 10-1 This image depicts a dog in right lateral recumbency with a large tumor involving the left axilla within which significant uptake of radionuclide is visible. The site of FDG injection is visible on the right antebrachium. Note normal uptake of radionuclide within the brain, salivary glands, myocardium, and kidneys. |
The recent fusion of PET with computed tomography (CT) has been an important step in maximizing the attributes of both modalities. 6-8 The fused scanner design allows anatomy and function to be assessed in a one-scan session with single positioning of the patient. This minimizes organ movement with no requirement for labor-intensive image registration algorithms as when the scans are obtained separately. 7,8 Accurately aligned fused images of anatomy and function obtained with PET/CT offer advantages to the study interpreter through accurate localization of tracer accumulation, the distinction of normal uptake of tracer within certain organs/tissues from pathologic uptake, and the verification that a suspicious finding on one modality can be confirmed by the other. 7,8
POSITRON EMITTERS AND PRACTICAL ASPECTS OF PET IMAGING
FDG and the Glycolytic Pathway
The most common radiopharmaceutical used in modern PET imaging is FDG. Developed in 1976 for the purpose of mapping regional cerebral glucose metabolism, this molecule is an analogue of glucose that is used to quantify the rate at which the hexokinase reaction of glycolysis is occurring in a tissue or an organ. 9 The development of FDG is based on the intracellular fate of 2-deoxyglucose (2-DG), an analogue of glucose that is phosphorylated in a similar manner by the hexokinase enzyme, the first step of glycolysis. However, once phosphorylated, 2-DG-6-P is trapped within the cell, unable to undergo the ensuing steps of glycolysis or the pentose phosphate shunt. Phosphorylated FDG, as with 2-DG-6-P, cannot be further metabolized, so all accumulated radioactivity over time is proportional to the rate of the hexokinase reaction in the observed tissue. At steady state conditions, this represents the rate of glycolysis in the tissue. 9
Scanning Technique
Patient Preparation
In human oncology, routine patient preparation involves fasting for approximately 6 hours before FDG injection to maximize uptake of the tracer by the tumor. After injection, it is important that the patient remain still and quiet for 90 minutes while FDG uptake occurs to avoid active skeletal muscle uptake of FDG as an interpretive pitfall. 10 For veterinary patients, fasting and use of a sedative premedicant with cage confinement is recommended after FDG injection to minimize aberrant uptake of FDG in skeletal muscle. Generally PET scans, similar to CT or MRI, are performed under general anesthesia in veterinary patients.
Image Interpretation and Standardized Uptake Value
When evaluating a static PET image, the most common way to assess tracer uptake is with the standardized uptake value (SUV). 5 With the use of computer software, a region of interest (ROI) is drawn around a lesion or an organ of interest and the activity of the tracer in this area is measured. The SUV is calculated to determine the relative tracer uptake within the ROI. As with other nuclear medicine techniques, visual inspection of the images is also used to characterize suspect areas of increased tracer uptake.
Uptake of FDG is not specific to malignancy. Organs such as brain, liver, spleen, tonsils, thymus, salivary glands, urinary system, and bone marrow are known to have varying degrees of normal FDG uptake. 10 Inflammatory processes are known to exhibit increased glucose metabolism through chemotaxis and phagocytic activity of inflammatory cells. Most malignant diseases produce lesions with higher SUVs than inflammatory conditions, but a few exceptions, specifically diseases causing granulomatous inflammation, do exist. 11
FDG-PET IN ONCOLOGY
Human Applications
Applications of PET and PET/CT are numerous and widely visible in human oncologic practice. 1,3 The unique ability of PET to non-invasively assess the likelihood of malignancy based on the SUV value has resulted in improved detection and management of, for example, solitary pulmonary nodules that may represent adenoma, granulomas, or neoplasia. 12 However, PET is more often applied in the staging of malignancy and assessment of response to therapy than in the initial diagnosis of human cancers. FDG-PET has been applied to the staging and follow-up of cancer of the breast, lung, colon, brain, and Hodgkin’s disease (HD) and non-Hodgkin’s lymphoma (NHL), becoming the test of choice in the staging, assessment of response to therapy, and detection of recurrence in both human HD and NHL. 12-18
Veterinary Applications
Lack of available equipment and high cost of PET radiopharmaceuticals have limited the use of PET as a diagnostic tool in veterinary oncology. Reports of PET in animals are sparse in the veterinary literature. 11,19,20 PET was used to characterize experimentally induced and naturally occurring blastomycosis and was compared with cases of canine lymphoma. 11,20 A recent study of whole-body PET in normal dogs demonstrates patterns of 18 FDG distribution and SUVs for parenchymal organs to assist in lesion interpretation in disease states. 21 Currently, the availability of PET for staging and evaluation of response to therapy in clinical patients is limited to a few locations in the United States. Applications in veterinary oncology will increase as this technology becomes more widely available, aided by regional cyclotron distribution networks for radiopharmaceuticals. As studies are published that validate its use as a non-invasive whole-body staging method, PET will become a useful and innovative tool in the management of veterinary oncology patients.
Selected References ∗
G.J.R. Cook, E.A. Wegner, I. Fogelman, Pitfalls and artifacts in 18 FDG PET and PET/CT oncologic imaging , Semin Nucl Med 34 ( 2004 ) 122 ;
In response to increasing use of PET in human oncologic imaging, this paper does a very good job of detailing the potential artifacts and pitfalls in image interpretation for optimization of PET interpretation .
J.W. Friedberg, V. Chengazi, PET scans in the staging of lymphoma: current status , Oncologist 8 ( 2003 ) 438 ;
A clinician-friendly review of the current accepted usage of PET in the diagnosis and management of human lymphomas .
M.E. Juweid, B.D. Cheson, Positron-emission tomography and assessment of cancer therapy , N Engl J Med 354 ( 2006 ) 496 ;
This is a recent review of the common applications of PET and PET/CT in human oncology .
A.K. LeBlanc, B. Jakoby, D.W. Townsend, et al. , Thoracic and abdominal organ uptake of 2-deoxy-2-[18F]fluoro-D-glucose (18FDG) with positron emission tomography in the normal dog , Vet Radiol Ultrasound 49 ( 2 ) ( 2008 ) 182 ;
The first report of dynamic organ 18 FDG uptake in normal dogs to aid in lesion interpretation in future studies of canine diseases imaged with PET .
D.W. Townsend, Physical principles and technology of clinical PET imaging , Annals Acad Med Singapore 33 ( 2004 ) 133 ;
This comprehensive review of PET covers both basic physics and technical factors influencing image quality. It contains an in-depth discussion of scanner design and technical aspects of PET/CT fusion .
SECTION B Computed Tomography (CT) and Magnetic Resonance Imaging (MRI)
Craig A. Clifford, Anthony J. Fischetti, Justin M. Goggin and E. Scott Pretorius
KEY POINTS
• CT and MR images are two-dimensional tomographic slices, which allow the observer to evaluate an area of interest without interference from overlying structures that would be summed in planar radiographs.
• CT imaging is usually performed in the transverse (axial) plane, whereas MR images are routinely made in multiple planes.
• MR provides superior anatomic detail of the nervous system and is considered the modality of choice for neuroimaging.
• For thoracic imaging, CT is superior to MRI because of an overall lower cost, more widespread availability, faster imaging with fewer artifacts associated with cardiac and respiratory motion, and generally shorter anesthesia time.
• Both CT and MRI are considered the standard of care in human oncology for diagnosis, staging, and assessment of response to therapy for many malignancies, including those of the liver, as well as of the spleen, adrenal gland, kidney, and pancreas.
• High accuracy of CT for diagnosis of dogs with different sources of chronic nasal disease (neoplasia, non-specific rhinitis, fungal rhinitis) has been reported.
The use of CT and MR imaging in veterinary practice has grown rapidly over the past few years. Although standard radiography and ultrasound remain important in diagnostic evaluation, CT and MR have emerged as important tools for evaluation of patients in whom diagnosis, disease stage, or tumor response to therapy are not well-evaluated by other means.
CT and MR images are two-dimensional tomographic slices that allow the observer to evaluate an area of interest without interference from overlying structures that would be summed in planar radiographs. 1-3 CT images are generally made in the transverse plane, whereas MR images are made in any plane. CT’s spatial resolution is greater than that of MRI, although MRI has the highest intrinsic contrast resolution of the three cross-sectional imaging modalities (ultrasound, CT, and MRI). 3 CT attenuation values are reported on the Hounsfield scale, which ranges from –1000 to +1000, with water assigned the value of 0. 3-5 There is no similar absolute scale for MR, since MR signal intensity values depend upon a large number of scanner and pulse sequence parameters. Valid comparisons between signal intensity measurements can therefore only be made within a given pulse sequence acquisition, or between two data acquisitions (such as pre- and post-contrast datasets) obtained with exactly the same scan parameters as one another.
The basic physical principles of radiography apply to CT image acquisition, although a volume of tissue receives a larger radiation dose in CT than with radiography. 5 Differential X-ray absorption by tissues is used to generate an image on a radiograph or, in CT, these absorption/transmission data can be mathematically interpolated by a computer, which then assigns a value in Hounsfield Units (HU) to each voxel within a CT slice. Since the human eye cannot distinguish 2000 shades of grey, CT data may be viewed in different “windows,” allowing the observer to assign visible shades of grey to a clinically useful range of HU. A window width is assigned to encompass all clinically relevant HU values, and a window “level” is the central HU value within the window. Window-level combinations in common clinical use include those for evaluation of the abdomen, brain, bones, and lungs.
MR imaging takes advantage of the magnetic properties of certain atomic nuclei, primarily protons (hydrogen ions), that are abundant within the body. At rest, the body’s protons are aligned randomly in space. Within the strong magnetic field of the MR scanner, protons line up either parallel or anti-parallel to the main magnetic field. A series of radio-frequency pulses—a “pulse sequence”—perturbs the protons from their equilibrium. When the radio waves are removed, the body’s protons tend to relax back to their alignment with the scanner’s magnetic field. They do so, however, at different rates within different chemical environments. These differential proton relaxation rates are monitored by detectors and are used to form the MR image. 3
NEUROIMAGING
Loss of symmetry, changes in tissue density (CT) or signal intensity (MR), displacement of normal structures, and changes in contrast enhancement are evaluated in the diagnostic evaluation of the brain or vertebral column. 5-7
MRI vs. CT
Both CT and MRI can assess loss of symmetry and displacement of normal tissues (a mass effect, termed like a “falx cerebri shift” in the brain). 5,6 However, MRI provides superior anatomic detail of the nervous system and is considered the modality of choice for neuroimaging. 5,8 Small lesions and lesions lacking contrast enhancement are less likely to be identified on CT. 9,10 Resolution is especially compromised when CT is used to assess the caudal fossa because of the dense surrounding petrous temporal bone, causing unwanted black streaking artifacts, called beam hardening . 11 CT-guided brain and retrobulbar biopsies have high diagnostic accuracy and low complication rates in dogs. 12-14
Brain and Spinal Tumors
Correlations between imaging characteristics and tumor types have been reviewed extensively. 6,7,15,16 Some claim excellent agreement between histologic diagnosis and imaging diagnosis. 16 Others express caution in defining tumor type based on imaging characteristics alone ( Table 10-1 ). 17,18 This hesitation is primarily due to the overlap of imaging signs for different tumor types and between neoplastic versus non-neoplastic diseases. 18,19 Disease prevalence, clinical presentation, and CSF analysis are often used in conjunction with imaging signs to support an imaging diagnosis. 20
| Note: “Dural tail” 22 sign; most specific for meningioma on contrast-enhanced T1-weighted MR images. 18 The dural tail is strong linear contrast-enhancement at the border of the tumor with the adjacent meninges because of vascular congestion in the dura mater, and not necessarily because of tumor invasion 23 (see Figure 10-2 , A ). | |||||
| The “Golf tee” sign is characteristic of intradural-extramedullary lesions of the vertebral column. The differential diagnosis for this sign includes but is not limited to meningiomas, nerve sheath tumors, and, depending on location/signalment, nephroblastoma. 23 | |||||
| Edema is non-specific and often peritumoral. Seizure-related edema can be seen with brain neoplasia. The edema is not restricted to the peritumoral region, can be symmetric, tends to affect the piriform/temporal lobe region, has variable contrast enhancement, and most importantly, will resolve once the seizure activity is resolved. Do not misinterpret seizure-related edema in a patient with a brain tumor as a multi-focal inflammatory or neoplastic disease process. 24 | |||||
| The intensity or density of the lesions has been purposefully omitted since this generally does not define a tumor type. Most lesions are isodense to hypodense on CT prior to contrast administration. Most lesions are hyperintense on T2-weighted MR with isointense to hypointense on T1-weighted MR. Variations in these generalities depend on the amount of edema, hemorrhage, mineralization, and cellular content. 6,7,9,10,18,25 | |||||
| ∗ Choroid plexus tumors include papillomas and carcinomas. Ependymoma should also be in the differential diagnosis for these imaging characteristics. 7,21 | |||||
| Meningioma | Choroid Plexus Tumor ∗ | Pituitary Neoplasia | Gliomas | Metastases | |
|---|---|---|---|---|---|
| Location | • Broadly based along calvarium, falx, or tentorium ossium < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


| ||||