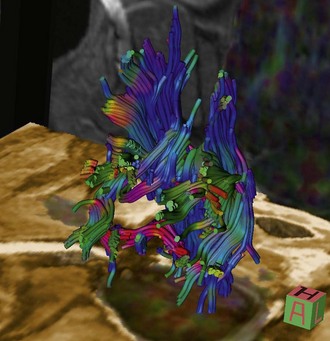
Chapter 15 Magnetic Resonance Imaging (MRI) There are a number of other MR imaging sequences that are becoming increasingly utililized in veterinary medicine. These include phase-contrast or CINE-MRI and diffusion-weighted imaging (DWI). Phase-contrast or CINE-MRI utilizes motion-sensitive pulse sequences in order to evaluate CSF flow. This type of imaging has potential for application in abnormal CSF flow disorders such as Chiari-like malformation with syringomyelia. Diffusion-weighted imaging is based on MR measurement of the diffusion of intracellular water. With ischemic and inflammatory conditions of the CNS, cytotoxic edema leads to abnormalities of water diffusion (damage to ATPase pumps) that can be visualized via DWI. This mode of MR imaging is particularly applicable to ischemic/vascular events and inflammatory disorders, as abnormalities can be detected earlier in the disease course compared with conventional (e.g., T2-weighted, FLAIR) MR sequences. A variation of DWI called diffusion tensor imaging (DTI) or MR tractography, is used to evaluate white matter tracts via evaluation of the direction of water diffusion along these tracts (Fig. 15-1).
Advanced Imaging for Surgeons
Physics: Overview
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine