Chapter 3 Acute Renal Failure
Introduction
A. Acute renal failure (ARF) is a clinical syndrome characterized by an abrupt increase in serum creatinine and blood urea nitrogen (BUN) concentrations to above normal (azotemia).
1. ARF also may be associated with abnormal regulation of electrolytes, acid-base, and fluid balances.
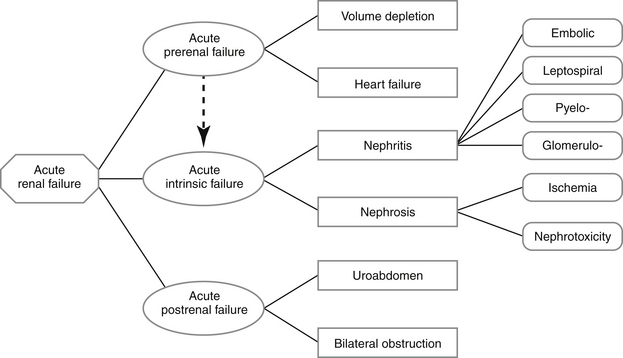
B. Acute renal failure can be prerenal, intrinsic (primary), or postrenal in origin.
1. The term acute renal failure is commonly used to refer to acute intrinsic renal failure (AIRF), but prerenal and postrenal causes also should be considered
2. Prerenal azotemia is the most important differential diagnosis to consider along with intrinsic causes of azotemia.
C. Early recognition of AIRF is crucial because it can be reversed in patients with enough surviving nephrons when treatment is instituted early on in the course of the disease.
D. AIRF probably occurs more frequently than appreciated and may go undiagnosed or be confused with chronic renal failure. Although AIRF can sometimes be successfully treated, many affected patients do not survive. Recognizing situations in which AIRF is likely to develop and taking appropriate preventive measures are preferable to treating established AIRF.
E. The frequency of underlying conditions associated with AIRF varies with the location and nature of veterinary practice.
1. Nephrotoxicity is the most common cause of AIRF at The Ohio State University Veterinary Hospital, followed by nephritis (e.g., leptospirosis) and ischemia.
2. The aggressive use of potentially nephrotoxic antibiotics, especially aminoglycosides, contributes to the frequency of nephrotoxic AIRF.
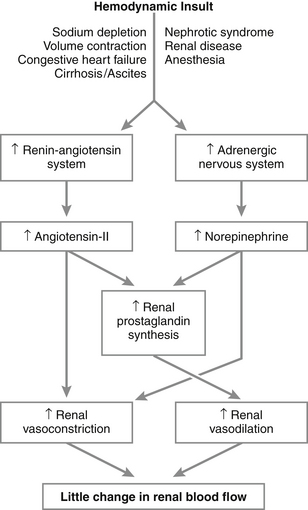
Pathogenesis and Pathophysiology of Acute Renal Failure (Figure 3-1)
Prerenal Acute Renal Failure
1. Reduced perfusion of the kidneys results in retention of nitrogenous wastes. A substantial decrease in hydrostatic pressure in the glomeruli results in decreased glomerular filtration rate (GFR) and solute retention.
2. Early in the process of volume depletion, renal autoregulation preserves excretory function and continued excretion of waste products. Autoregulation eventually is overcome and azotemia develops.
3. The most common causes of reduced renal perfusion are dehydration, hypotension, shock, and reduced cardiac output.
4. Early prerenal ARF is associated with physiologic oliguria (assuming normal kidneys initially), which is a normal response to decreased renal perfusion.
5. Decreased renal perfusion is detected in the kidney and by peripheral baroreceptors, and results in increased reabsorption of salt and water from the glomerular filtrate.
6. Severe and prolonged hypoperfusion of the kidneys can produce primary lesions in the kidneys (ischemic nephrosis or ischemic acute tubular necrosis).
7. Prerenal azotemia readily resolves after correction of decreased cardiac output and circulating blood volume.
Postrenal Acute Renal Failure (See Chapters 11, Urinary Tract Obstruction, and 12, Urinary Tract Trauma and Uroabdomen)
Acute Intrinsic Renal Failure (primary ARF)
Causes of Acute Intrinsic Renal Failure (see Chapter 4, Specific Syndromes Causing Acute Intrinsic Renal Failure)
Pathophysiology of Acute Intrinsic Renal Failure Due to Nephrosis
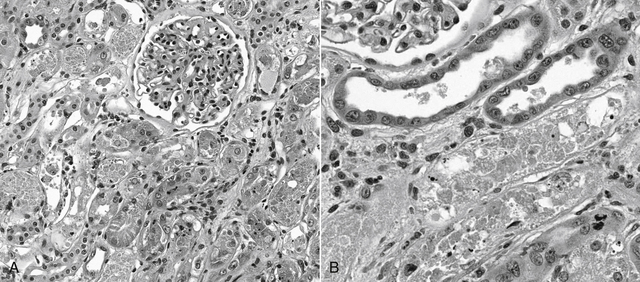
a. Exposure to nephrotoxins or ischemia causes tubular injury along a spectrum from degeneration to necrosis and is referred to as nephrosis or acute tubular necrosis (ATN) (Figure 3-2). Some patients have minimal to no light microscopic lesions but experience severe renal excretory failure.
(1) Early lesions may be distributed preferentially in tubules of the outer medulla, an area that is not easily biopsied.
(2) Renal tubular lesions may be subtle in nature and patchy in distribution making them easy to overlook on routine microscopy.
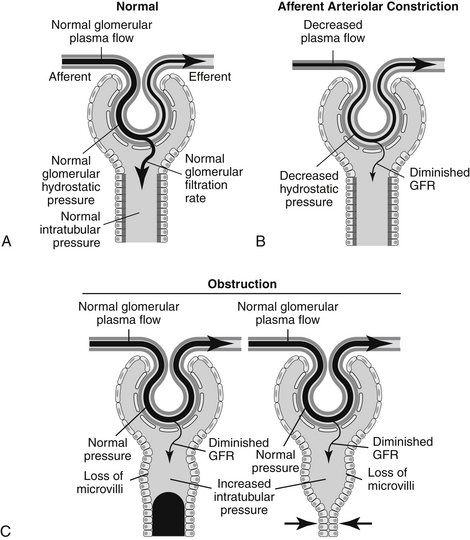
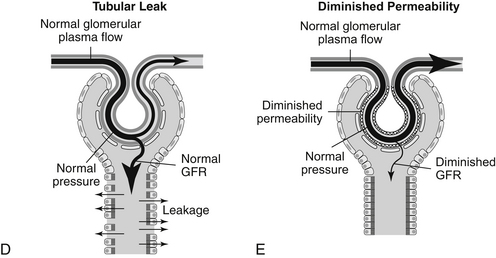
b. Factors that contribute to azotemia and oliguria during AIRF (Figure 3-3 A-E).
c. The mechanism(s) that initiates AIRF may not be the same one(s) that maintains AIRF. A combination of mechanisms likely is operative in clinical patients.
d. Renal ischemia consists of a spectrum from prerenal azotemia to acute tubular necrosis and in its most devastating form, bilateral cortical necrosis.
(1) The renal cortex is richly supplied with adrenergic innervation, which results in vasoconstriction during renal ischemia. Due to a large reserve of blood supply, temporary or mild reductions in renal blood flow do not result in tubular necrosis.
(2) Deprivation of blood supply, if severe and prolonged, results in loss of cellular energy production and loss of cell integrity. Tubules with high metabolic activity are at greatest risk of injury during reduced oxygen supply.
(a) It is not necessary for systemic hypotension to occur for the kidneys to experience intrarenal hypotension.
(b) The outer medullary region of the kidney is the most metabolically active and is supplied with the lowest amount of oxygen. The last part of the proximal tubule (pars recta, P3) and the medullary thick ascending limb of the loop of Henle are located here and these areas are at increased risk for early injury during hypoxia. The medulla already is an area of low blood supply that is at increased risk when blood flow is further reduced.
e. NSAIDs block renal production of vasodilatory prostaglandins that maintain renal blood flow during dehydration resulting in renal ischemia (Figure 3-4).
f. True nephrotoxins exert their deleterious effects directly on the kidney after binding to tubular cell membranes. Reduced energy production and cell death follow. Some nephrotoxins also cause renal vasoconstriction, but the major effect is direct cell injury rather than ischemia.
g. The term nephrotoxicant refers to a chemical or drug that can result in renal injury regardless of whether it is by direct nephrotoxic injury (e.g., aminoglycosides) or by renal ischemia (e.g., NSAIDs, myoglobin).
h. Patients with underlying renal disease develop episodes of AIRF more readily than patients that had normal kidneys before the insult.
(1) The ability of diseased kidneys to autoregulate blood flow and GFR is decreased, placing them at greater risk for renal ischemia. They cannot undergo compensatory vasodilatation to compensate for systemic hypotension.
(2) The ability to synthesize renal vasodilatory prostaglandins is reduced, placing them at risk for renal ischemia.
(3) The increased tubular metabolic rate of remnant nephrons places them at risk due to their increased workload. They are more readily injured during ischemia and exposure to toxins.
(4) Increased single nephron GFR in remnant nephrons provides an increased filtered load of potential toxins per remaining nephron.
i. Dehydration magnifies the propensity for and severity of AIRF after exposure to renal ischemia or nephrotoxins.
(1) Dehydration promotes maximal water and solute reabsorption along the nephron. Toxins that are filtered across the glomeruli progressively increase in concentration along the course of the proximal tubule as water and sodium are iso-osmotically reabsorbed.
(2) The slow tubular flow rate associated with dehydration favors formation of obstructing casts after renal ischemia or exposure to a nephrotoxin.
j. Loss of integrity of the cytoskeleton of the renal tubular cell and actin dysregulation are pivotal early events in nephrosis. Loss of microvilli occurs with loss of the cytoskeleton. Cell adhesion is impaired at tight junctions (loss of occludin) and along basolateral membranes (loss or redistribution of integrin). These events facilitate detachment of tubular cells from one another and from the tubular basement membrane. Relocation of Na+-K+ adenosine triphosphatase (ATPase) from the basolateral to luminal cellular membranes impairs sodium reabsorption and results in activation of the renin-angiotensin-aldosterone system and afferent arteriolar vasoconstriction (i.e., vasomotor nephropathy). Depletion of intracellular adenosine triphosphate (ATP) triggers a cascade of events that alters cell membrane permeability, increases calcium influx, and impairs cell function ultimately causing in cell death. The balance between nitric oxide and endothelin, which maintains normal renal blood flow, is altered (decreased nitric oxide and increased endothelin) favoring renal vasoconstriction. Damaged myocytes of afferent arterioles allow calcium influx which potentiates vasoconstriction. Growth factors (e.g., epidermal growth factor, hepatocyte growth factor, insulin-like growth factor, transforming growth factor) and heat shock proteins appear to be important in recovery from AIRF.
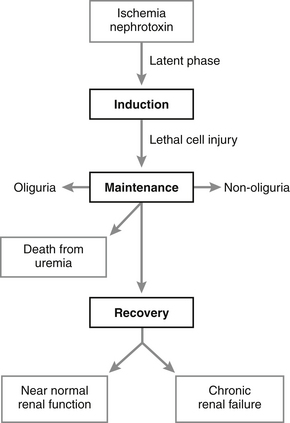
k. Phases for AIRF (Figure 3-5).
(1) The latent phase represents the time after exposure to a nephrotoxin or renal ischemia but before the onset of azotemia. It is associated with increasing number and severity of renal tubular lesions over time if the renal insult is not stopped.
(c) Early removal of the inciting cause of injury will result in rapid return to normal renal function.
(d) The latent phase is most likely to be detected in closely monitored hospitalized patients with critical illness.
(e) Older definitions of AIRF required that oliguria be documented during the clinical course, but this is no longer a requirement. Oliguria, normal urine production, or polyuria all may occur depending on the specific cause and severity of renal injury and the phase of AIRF.
(2) The maintenance phase of AIRF is characterized by a persistently increased serum creatinine concentration despite correction of all prerenal factors (i.e., restoration of extracellular fluid volume and cardiac output).
(a) Oligo-anuria occurs in patients with the most severe intrarenal injury (e.g., those with EG or lily toxicity).
(c) Entry into the maintenance phase signifies that a critical amount of lethal injury has occurred in the renal tubules and a course of 1 to 3 weeks of AIRF is expected before restoration of renal function can occur.
(e) Renal injury may be so severe that it may not be possible for the patient to ever leave the maintenance phase.
(3) During the recovery phase, BUN and serum creatinine concentration return to normal as GRF and RBF recover in animals capable of surviving the maintenance phase. Functional renal mass, however, has been reduced.
(b) Complete return of BUN and serum creatinine concentrations to normal may not be possible for patients that have sustained the greatest renal injury. They may, however, show partial improvement that will allow a reasonable quality of life as a chronic renal failure patient.
(d) Remaining renal tissue is not completely normal even when BUN and serum creatinine concentrations have returned to normal. Some nephrons invariably have been lost and replaced with fibrous tissue. Renal hypertrophy and hyperplasia have enabled the kidney to restore some RBF and GFR with fewer remaining surviving nephrons.
History and Clinical Signs
Postrenal Acute Renal Failure (see Chapter 11, Urinary Tract Obstruction, and Chapter 12, Urinary Tract Trauma and Uroabdomen)
Acute Intrinsic Renal Failure
1. Clinical signs are those of uremia, including anorexia, lethargy, vomiting, and diarrhea. These signs are not specific for AIRF.
2. Signs will be of recent onset, and a longstanding history of polyuria or polydipsia should not be present.
3. Based on clinical studies, approximately 18% of affected patients are expected to have anuria, 43% to have oliguria (urine output of 0.1-1 mL/kg/hr), 25% to have normal urine output (1-2 mL/kg/hr), and 14% to have polyuria (urine output >2 mL/kg/hr).
4. Lack of urinations may be noted in animals with oliguric AIRF or polyuria may be noticed in those with nonoliguric ARF.
5. Recent trauma, shock, surgery, or anesthetic procedures suggests the possibility of ischemic nephropathy.
6. Recent administration of known nephrotoxins (e.g., aminoglycosides) should lead to a suspicion of nephrotoxic AIRF.
7. Recent administration or accidental ingestion of NSAIDs should lead to a suspicion of ischemic AIRF.
8. Access to EG or actual observation of EG consumption indicates nephrotoxic AIRF, but often a positive history cannot be obtained.
9. Postural changes (e.g., hunching of the back) or reluctance to move may be indicative of renal pain.
Physical Examination
Postrenal Acute Renal Failure (see Chapter 11, Urinary Tract Obstruction, and Chapter 12, Urinary Tract Trauma and Uroabdomen)
Acute Intrinsic Renal Failure
a. Signs of uremia tend to be more severe than those observed with prerenal azotemia, and include uremic breath and oral ulceration.
d. Hypothermia is commonly encountered in animals with ARF due to nephrosis, but hypothermia can be encountered in any animal near death, regardless of the cause of azotemia.
e. Dehydration frequently is present in animals with primary AIRF due to fluid losses from vomiting, diarrhea, and lack of food and water intake. Dehydration may be exacerbated by excessive urinary loss of fluid in animals with nonoliguric or polyuric AIRF.
< div class='tao-gold-member'>
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree