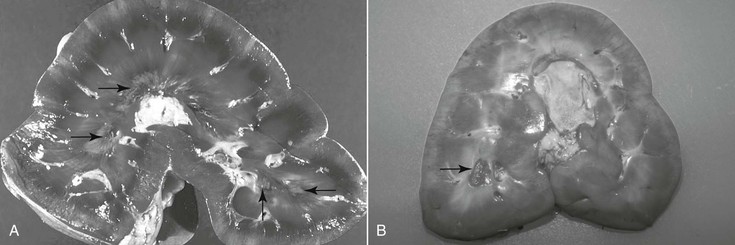
Harold C. Schott II, Prerenal failure has long been used to describe an acute, reversible increase in nitrogenous waste products (azotemia) in the bloodstream, associated with a transient decrease in renal function secondary to renal hypoperfusion. Although this term is entrenched in both the human and veterinary medical literature, its use likely contributes to the lack of recognition of subclinical renal damage that may accompany a number of medical problems. This can be attributed to the large reserve capacity of the kidneys, in which nearly 75% of nephron function must be compromised before dysfunction is recognized clinically. With prerenal failure, adequacy of renal function is characterized by maintenance of concentrating ability (urine specific gravity >1.035) and normal serum and urine electrolyte concentrations. Unfortunately, urine samples are rarely collected from horses at the time of hospital admission to document specific gravity: urine tonicity declines and urine Na+ concentration increases rapidly with supportive fluid therapy. As a consequence, it can be challenging to distinguish between prerenal failure and intrinsic renal damage (renal azotemia, for which loss of concentrating ability is a hallmark) by measuring urine specific gravity in horses that have been receiving fluids for 6 to 12 hours. Nevertheless, suspicion of changes in glomerular and tubular function and integrity can be supported by detecting pigmenturia, proteinuria, or glucosuria with reagent strip analysis or cast formation on microscopic examination of urine sediment. Despite the reversible nature of azotemia and urinary alterations with prerenal failure, nephron injury (and a degree of nephron loss) likely occurs in most instances of prerenal failure. To increase awareness of subclinical renal damage in patients with decreased renal blood flow (RBF) and glomerular filtration rate (GFR), the term acute kidney injury (AKI) has been introduced in human and, subsequently, small animal medicine. Acute kidney injury is defined as an increase in serum creatinine concentration of as little as 0.3 mg/dL (25 µmol/L) or a 50% increase from the baseline value, yet creatinine may remain within reference range, and serum electrolyte concentrations are usually normal. Furthermore, the mild increase in creatinine is reversible with appropriate supportive treatment. When AKI progresses to overt acute renal failure (ARF), abnormalities in serum electrolyte concentrations (hyponatremia, hypochloremia, and occasionally hyperkalemia), more substantial azotemia (creatinine >2.5 mg/dL or >220 µmol/L), loss of concentrating ability (urine specific gravity <1.020), and clinical signs of renal failure may become apparent. In the author’s hospital, the incidence of significant azotemia is slightly under 5% for horses that have a serum chemistry profile performed as part of their evaluation; however, mortality rate is high for horses with severe azotemia (creatinine >10 mg/dL or 880 µmol/L), with the exception of neonates with spurious hypercreatininemia (Table 110-1). TABLE 110-1 Incidence of Azotemia (and Associated Mortality Rates) in Horses Presented to a Veterinary Teaching Hospital (1997 and 2000) * Assumes that horses that did not have a serum chemistry performed also did not have azotemia. † The two survivors were neonatal foals with spurious hypercreatininemia. Acute kidney injury usually develops as a complication of another disease process or activity that leads to hypovolemia and a prolonged period of decreased RBF and GFR (e.g., colic, enterocolitis, hemorrhage, or endurance exercise). Acute kidney injury may progress to ARF when renal hypoperfusion persists or renal damage is exacerbated by exposure to nephrotoxic agents. Nephrotoxins that can cause AKI and ARF include endogenous pigments (myoglobin or hemoglobin), vitamin D or vitamin K3, heavy metals (e.g., mercury, cadmium, zinc, arsenic, and lead), and acorns (tannins). Treatment with nephrotoxic medications, including nonsteroidal antiinflammatory drugs (NSAIDs), aminoglycoside antimicrobials, or oxytetracycline (most commonly when administered for correction of flexural deformities in neonatal foals), remains a significant risk factor for development of iatrogenic ARF in horses. Hemodynamically mediated AKI/ARF is often associated with oliguria (urine output <0.5 mL/kg for longer than 6 hours), whereas urine production with nephrotoxin-associated AKI/ARF often remains normal (nonoliguric AKI). In neonates, ARF can also develop as a complication of septicemia, particularly with disseminated Actinobacillus equuli infection. Acute renal failure can also develop with Leptospira spp infections in equids. Most horses do not experience appreciable adverse effects from NSAID use as long as the drugs are administered at the proper dosage and treated animals are not dehydrated. When RBF decreases as a consequence of dehydration or diversion of cardiac output away from the kidneys (as during exercise), vasodilatory prostaglandins (PGE2 and PGI2) are produced within the kidney. Production of renal prostaglandins through cyclooxygenase (COX) pathways is greatest in medullary tissue, the region of the kidney that normally has the lowest blood flow and functions in a relatively hypoxic environment. Thus it should not be surprising that the lesion associated with NSAID use is inner medullary (medullary crest or papillary) necrosis (Figure 110-1). As with adverse gastrointestinal effects of NSAIDs, it appears that certain individuals are more sensitive to the adverse renal effects of these drugs. Consequently, the author prefers use of “NSAID sensitivity” rather than “NSAID toxicosis” when adverse effects are observed with NSAID treatment. Recent development of more COX-2–selective NSAIDs has received considerable attention, and it would be logical to assume that these NSAIDs may be less nephrotoxic than nonspecific NSAIDs. However, this new generation of COX-2–selective NSAIDs has not been demonstrated to be renoprotective in studies in other species, and their use should not be assumed to be renoprotective in equids. Aminoglycoside antimicrobials accumulate within proximal tubular epithelial cells during repeated administration of these drugs. After toxic amounts are sequestered within the tubular cell, cellular metabolism is disrupted, and cell swelling, death, and sloughing into the tubular lumen occur. Most cases of aminoglycoside nephrotoxicosis are not the result of overdosing or administration of the drug to an azotemic patient. In fact, healthy kidneys can usually tolerate a single major overdose (10 times the normal dose) without detrimental effects. Similarly, delaying administration of the initial dose of an aminoglycoside antimicrobial until dehydration is corrected is unlikely to be renoprotective and may compromise treatment of sepsis. If initial laboratory data reveal moderate azotemia (e.g., creatinine >5 mg/dL or >440 µmol/L), consideration of an alternative to aminoglycoside antimicrobials for treatment is warranted, but a single previously administered therapeutic dose of an aminoglycoside is unlikely to significantly exacerbate AKI. When aminoglycosides must be administered to high-risk patients (those with persistent subclinical dehydration or sick neonates) for treatment of specific bacterial infections, volume deficits must be minimized, and creatinine should be monitored closely. In practice, nephrotoxicosis more commonly develops with repeated administration of the drugs for a week or longer during treatment of pleuropulmonary or musculoskeletal infections. Affected horses often appear adequately hydrated and maintain a reasonable appetite. Thus development of moderate azotemia, indicative of ARF, can be a surprise when serum chemistry analysis is performed as part of patient monitoring. This finding would warrant consideration of alternative antimicrobial drugs as well as discontinuation of NSAIDs. Because tubular absorption and accumulation of aminoglycosides is directly related to their serum concentrations, the current standard practice of once-daily administration has reduced the risk for nephrotoxicosis, compared with the multiple daily dose regimens that were used in the past. Because aminoglycoside antimicrobials have a concentration-dependent action against bacteria, once-daily dosing both ensures a higher peak serum concentration for antibacterial action and allows for a longer period during which the drug concentration lies below the trough value. Because renal tubular damage is usually sustained only when the drug is above the trough concentration, once-daily dosing can be considered renoprotective. Finally, in high-risk patients, repeated urinalysis at 2- to 3-day intervals may be warranted to detect early tubular damage by finding changes in urine protein excretion or increased urine γ-glutamyltransferase (GGT) activity. Proximal tubule apical membranes have a highly developed brush border that is rich in GGT, and activity of this enzyme, often expressed as a ratio to urine creatinine (normal value for GGT/creatinine is <25), increases in the urine as tubular epithelial cells are sloughed into the lumen. This urine biochemical abnormality may be detected several days before the onset of azotemia; however, many clinicians consider this test to be too sensitive to support discontinuation of aminoglycoside administration when a drug of this class is indicated for treatment of a given infection. An alternative to discontinuing use of these drugs may be to further extend the dosing interval to 36 to 48 hours after the initial 7 to 10 days of once-daily administration.
Acute Kidney Injury
Evolution of Terminology and Incidence of Acute Renal Failure
1997
2000
Number of horses examined
1902
2289
Number (and %) of horses on which serum chemistry was performed at admission*
397 (21%)
423 (18%)
Number (and incidence, %) of horses with Cr >2.5 mg/dL
82 (4.3%)
81 (3.5%)
Number (and incidence, %) of horses with Cr >5 mg/dL [mortality rate]
15 (0.8%) [31%]
19 (0.8%) [44%]
Number (and incidence, %) of horses with Cr >10 mg/dL [mortality rate]
2 (0.11%) [100%]
3 (0.13%) [33%]†
Number of horses with primary renal disease
3 (0.16%)
2 (0.09%)
Causes of Acute Kidney Injury and Acute Renal Failure
Nonsteroidal Antiinflammatory Drugs
Aminoglycoside Antimicrobials
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree