CHAPTER 29 Acromegaly
ETIOLOGY
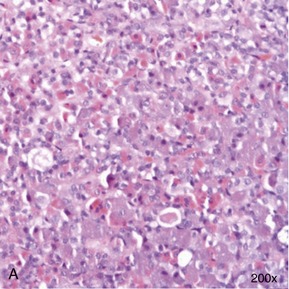
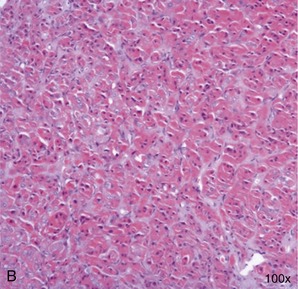
In contrast to the situation in dogs, in whom we encounter acromegaly mostly when induced by exogenous or endogenous progestogens, acromegaly in cats traditionally is thought to be caused by a functional somatotrophic adenoma in the pars distalis of the anterior pituitary gland, resulting in excessive growth hormone (GH) secretion1 (Box 29-1). However, a recent report suggests that a proportion of acromegalic cats are suffering from somatotrophic hyperplasia rather than a specific adenoma2 (Figure 29-1). Whether this represents a different group of acromegalics, or a rare subset of the same spectrum of disease, remains to be determined. Additionally, one case with a double pituitary adenoma causing both hyperadrenocorticism and acromegaly has been described.3 Which processes initiate pituitary adenoma formation remain to be elucidated.
Box 29-1 Brief Overview of Feline Acromegaly
The expression of GH mRNA in mammary gland tissue, as seen in dogs, also can be found in cats and human beings, indicating that secretion of GH from mammary tissue also could occur in cats. Indeed, progestin-induced fibroadenomatous changes in the mammary glands of cats also are associated with locally enhanced GH expression. The mammary gene is identical to the pituitary-expressed gene and uses the same promoter for transcription. Nevertheless, feline cases of progestin-induced GH secretion from the mammary glands causing the clinical syndrome of acromegaly have yet to be described.4,5
EPIDEMIOLOGY
Acromegaly in cats previously was thought to be rare, with only approximately 30 cases reported in peer-reviewed literature before 2007.6,7 Recent research has attempted to provide a more accurate insight into the possible true prevalence of this endocrinopathy. A large general screening study in the United Kingdom among diabetic cats with variable glycemic control (ranging from poor to adequate) who were not suspected initially to have acromegaly revealed that 59 out of 184 assessed subjects (i.e., 32 per cent of screened cats) had serum insulin-like growth factor 1 (IGF-1) concentrations strongly suggestive of the presence of acromegaly (Figure 29-2).2 Of these 59 cats a subpopulation of 18 cats was assessed in more detail, leading to confirmation of the diagnosis in 94 per cent of assessed cases. Confirmation of acromegaly in such a high proportion of the subset suggests that the original estimation of prevalence in the 184 cats in the study, made on the basis of raised IGF-1 levels only, was likely to be an accurate approximation of the true prevalence in the greater diabetic population. Additionally, the data from this prospective British study were backed up by data from a retrospective North American study, which indicated that 26 per cent of diabetic cats met clinical criteria for the diagnosis of acromegaly.8 The latter study did, however, include samples of diabetic cats that were submitted to the laboratory specifically for assessment for acromegaly, introducing a bias to the study. Overall, it remains a genuine possibility that we are currently underdiagnosing acromegaly and revealing only the tip of the iceberg.9
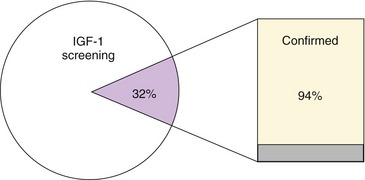
Figure 29-2 The results of a large prospective British screening study among diabetic cats.2 Pie chart: 32 per cent of screened diabetics had an IGF-1 > 1000 ng/mL (purple segment), consistent with acromegaly. Bar chart: further evaluation of a subgroup of these 32 per cent (n = 18) confirmed acromegaly in 94 per cent (yellow segment) on the basis of intracranial CT, MRI, and/or postmortem findings.
In early reports acromegalic cats typically were middle-aged to older, neutered male mixed-breed cats with insulin-resistant diabetes mellitus.1,6,10–14 Recent reports have confirmed this signalment, although insulin resistance was frequently but not consistently present at time of initial diagnosis. The authors found that of 59 cats with IGF-1 concentrations consistent with acromegaly, 52 were domestic shorthair cats, three domestic longhair, and four of unknown breed. Forty-seven were neutered males; six neutered females; five intact males; and one was of unknown gender. Their age ranged from 6 to 17 years (median, 11 years); body weight ranged from 3.5 to 9.2 kg (median, 5.8 kg), and median insulin dosage was 7 U per cat q12h (range, 1 to 35 U q12h), compared with 3 U per cat q12h in cats with IGF-1 concentrations below the acromegaly range.2 However, because relatively few cases have been documented to date, it seems prudent that clinicians remain open-minded with regard to this signalment.
PATHOGENESIS
Somatotrophic adenomas (and possibly somatotrophic hyperplasia) result in an increase in amplitude, duration, and frequency of the pulsatile release of GH, which in turn leads to elevated production of IGF-1, predominantly by the liver.15 Affected cats suffer the catabolic and diabetogenic effects of GH, the anabolic effects of IGF-1, and, in some cases, the neurological consequences of an expanding intracranial neoplasm. A GH-induced postreceptor defect in insulin action at the level of target tissues is thought to explain why most reported cats with acromegaly have had concurrent insulin-resistant diabetes mellitus.* High IGF-1 concentrations induce excessive soft tissue growth, as evidenced by renal and myocardial pathology, hepatomegaly (although this is likely to be partly a result of hepatic lipid accumulation), adrenomegaly, and incidental thyroid enlargement, in addition to bone remodelling and thickening, resulting in arthropathy, broad facial features, and enlarged (so-called clubbed) paws.
In light of the change in renal size, renal disease is understood to be a possible complication in long-term untreated acromegalics. Abnormalities in renal histopathology reported in acromegalic cats include diffuse thickening of the glomerular basement membrane, thickening of Bowman’s capsule, periglomerular fibrosis, and adipose and hydropic change with epithelial degeneration and regeneration of tubules.2
Pancreatic disease was reported previously by Gunn-Moore in two of 10 feline acromegalics,6 and also was evident in two cats who underwent postmortem examination in the authors’ study.2 In the latter study the pancreas of one cat appeared grossly enlarged and firm and contained two cysts; on histopathological examination, diffuse hyperplasia with fibrous tissue, numerous interstitial lymphoid follicles, and marked vacuolation of the islet cells were noted. It remains to be determined if pancreatic disease is indeed more common in acromegalic diabetic cats, especially in view of the small number of cases in whom pancreatic pathology has been reported specifically and the potentially high prevalence of pancreatic disease in diabetic cats.17
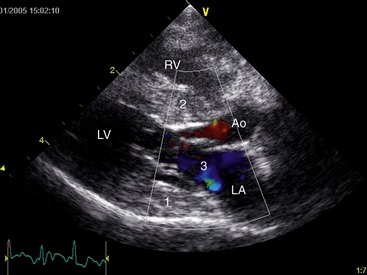
Echocardiography in confirmed acromegalic cats has revealed variable changes ranging from no indication of structural abnormalities to atrial enlargement, generalized or focal interventricular septal thickening, and left ventricular free wall hypertrophy with thinning towards the apex. Diastolic dysfunction, systolic anterior motion of the mitral valve (SAM), mitral valve insufficiency, and increased left ventricular outflow velocity, as well as poor left atrial wall motion, spontaneous echo contrast, and a restrictive pattern of pulmonary venous flow all have been reported (Figure 29-3).2 Overall myocardial changes seem common in acromegalic cats, and subsequent congestive heart failure can result. Kittleson et al reported increased GH concentrations among 31 cats with hypertrophic cardiomyopathy (HCM) when compared with 38 normal cats and 35 cats with cardiac disease of other cause. The serum GH concentrations in cats with HCM were less than those reported previously in cats with acromegaly. Pituitary tumors were not identified in eight of these cats with HCM on postmortem examination; however, presence or absence was not established for the remaining HCM cats.18 These findings are supportive of a possible direct causal role of GH in the myocardial changes encountered in acromegaly.
Although hypertension has been regarded as common in acromegalic cats,7,11 the largest and most recent study did not detect an increased prevalence of hypertension in acromegalics.2 If indeed present in individual acromegalic cats, hypertension may result in (and, therefore, cause a cat to be presented for) ocular hemorrhage, sudden blindness, or neurological abnormalities (see Chapter 49).
CLINICAL SIGNS
The soft-tissue and skeletal changes induced by IGF-1 have the potential to lead to a marked change in the overall or general appearance of an acromegalic cat. However, a recent study suggests the typical acromegalic phenotype is not particularly common.2 Certainly the absence of a typical acromegalic appearance should not decrease a clinician’s index of suspicion for the condition. Additionally, similar to the situation in human acromegaly, the feline disease has a gradual onset, often leading to late, or absence of, recognition of conformational changes by people in continuous contact with affected cats. Frequently comparison with pictures from past years is necessary to determine the difference in appearance and convince owners of these changes (Figure 29-4). Duration of the clinical signs (as perceived by the owners) in the cats seen by the authors indeed ranged broadly from 2 to 42 months (mean ± SD, 11.2 ± 11.4). In the authors’ study the earliest clinical signs reported by the owners (Box 29-2) included those consistent with diabetes mellitus (i.e., polyuria/polydipsia [100 per cent of cats] and polyphagia [94 per cent]), but also weight gain (59 per cent). Some cats developed lameness (29 per cent), and some eventually displayed suspected or confirmed neurological signs, lethargy, impaired vision, circling, and vocalizing.2 Initial weight loss followed by weight gain also has been reported.1 Owners of the confirmed acromegalic cats in the authors’ study had noted increased size of the paws in 12 per cent of cats, broader facial features in only one cat, and increased abdominal size in one cat. All cats were being treated with insulin at the time of diagnosis and all experienced difficulties in glycemic control, necessitating administration of increasing amounts of insulin before or after confirmation of the diagnosis of acromegaly. The median insulin dosage of the 17 confirmed acromegalic cats at time of initial examination in the study was 7 IU per cat q12h (range, 2 to 35 U q12h), higher than the 3 IU q12h for cats with IGF-1 below 1000 ng/mL.2 Additional reported clinical signs include intermittent weakness (hypoglycemia) and behavioral changes.1
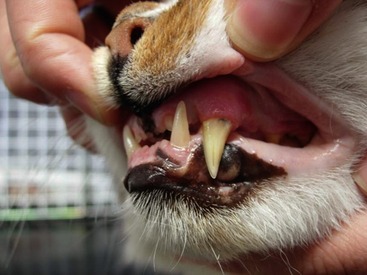
On physical examination of the 17 confirmed acromegalic cats, the authors noted enlarged liver and kidneys in 88 per cent, prognathia inferior (Figure 29-5) with increased distance between upper and lower canine teeth in 47 per cent, immature bilateral cataracts in 12 per cent, clubbed paws in 18 per cent (Figure 29-6), broad facial features in 82 per cent (see Figure 29-4), and systolic cardiac murmur in 24 per cent. They also noted gallop rhythm in one cat; respiratory stridor in 53 per cent; multiple limb lameness in 29 per cent; impaired vision, dilated pupils, reduced pupillary light reflex, reduced menace response, decreased facial sensation on the left and decreased postural reactions on the left, circling to the right, and vocalizing in one cat; decreased menace response bilaterally, confusion, and right-sided Horner’s syndrome in another; and plantigrade stance of the hind limbs also in one patient. One cat experienced periods of open-mouth breathing and tachypnea when stressed which was attributable to congestive heart failure. Feldman and Nelson additionally reported the presence of a large tongue, increased interdental spacing, poor and unkempt hair coat, dullness, and increased soft tissue of the pharyngeal region in individual acromegalic cats.1
DIAGNOSIS
Confirmation of acromegaly can be difficult because of the disorder’s insidious onset, the cost of imaging procedures, and the frequent lack of a readily available feline growth hormone (fGH) assay.1,19 As is the case in many diseases and specifically endocrinopathies, a combination of a suggestive clinical phenotype and indicative diagnostic aids is required to obtain an adequate level of confidence for a diagnosis of acromegaly (Box 29-3).