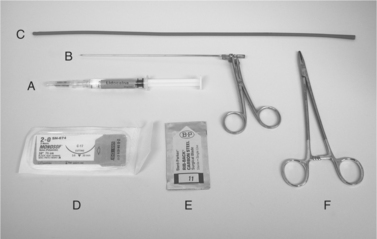
A
Abdominal Drainage
OVERVIEW AND GOAL
To evacuate large-volume ascites via percutaneous placement of a sterile tube into the abdomen
EQUIPMENT, ANESTHESIA
• Manual restraint; sedation needed only rarely. Note: Animals often are restless during restraint owing to pressure of ascites on diaphragm; in such cases, the procedure may be preceded by large-volume needle centesis or may need to be performed with the animal in standing position.
• Use 0.5-15 mL 2% lidocaine for local anesthesia. The volume is based on body weight, 1-70 kg. Discomfort from lidocaine infusion can be reduced by adding 0.05-0.2 mL sodium bicarbonate for injection (8.4% = 84 mg/mL = 1 mEq/L) and by warming solution to body temperature (armpit method).
POSSIBLE COMPLICATIONS AND COMMON ERRORS TO AVOID
• Overall, complications very uncommon; approximately 5%-10% incidence of irritation or dehiscence of incision, responding to topical treatment. Major complications are <1%.
• Concerns for hypovolemia, hypotension, hypoalbuminemia, and ascending bacterial peritonitis appear unjustified, given lack of occurrence in large case series (unpublished data).
• Elizabethan collar must be on the animal at all times during drainage to avoid self-induced damage to the tube.
• Once in place in the standing animal, the tube ± stopcock may be caught in the grate flooring of the cage when the animal lies down. Covering the grate on the floor of the cage with towels helps prevent this complication.
PROCEDURE
• Clip hair widely (ventral abdomen), with umbilicus approximately at center of clipped area. Since the procedure does not require surgical draping, long hair must be trimmed back extensively.
• Wide surgical scrub and prep, centered on ventral abdominal midline and just cranial to the umbilicus.
• Lidocaine infusion at planned point of entry; on abdominal midline, generally just cranial to the umbilicus. Use multiple (e.g., 6-8) small subcutaneous boluses.
• Note: More cranial is better in most males (greater distance from prepuce, so less risk of contamination), but it is important to avoid too cranial in animals with hepatomegaly (right-sided heart failure, some liver diseases).
• Note: Lidocaine infusion must be wide (cranial-caudal, left-right) and deep (reaching all layers, including peritoneum) and involves injecting many small pockets of lidocaine throughout the region of the planned incision.
• Caution: When redirecting, avoid subcutaneous tissue laceration with the needle tip. After each infiltration of a small pocket of lidocaine, partially withdraw the needle, redirect it, and then readvance it for the next infiltration. The multiple infiltrations can all be accomplished via a single point of needle entry (i.e., radial redirection of the needle).
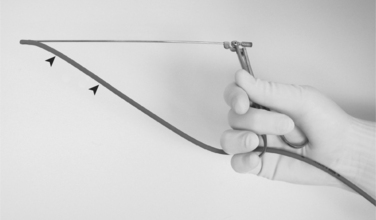
• After opening the sterile gloves, keep the paper wrapper flat and use as a sterile surface. Wear the sterile gloves from this point onward. Use the suture scissors for making three to five additional drainage holes in the red rubber feeding tube to avoid omental plugging during drainage.
• Note: To make extra holes prior to inserting the tube into the abdomen, kink the red rubber feeding tube with thumb and forefinger and snip off the corner of the folded edge; unfolded edge reveals a small oval hole (needs to be <50% of tube’s circumference to prevent weakening it). Repeat to make several holes along the distal half of the tube. The suture scissors are kept sterile for later in the procedure.
• Using the #11 scalpel blade, make a stab incision cranial to the umbilicus on the ventral abdominal midline at the center of the lidocaine-infiltrated area.
• Note: To avoid an excessively large incision, hold the #11 scalpel blade between the thumb and forefinger. The point at which the blade is held between the thumb and forefinger leaves a maximal width of the exposed blade that is the same as, or just slightly greater than, the diameter of the red rubber feeding tube to be inserted; that is, the fingertips act as a guard to prevent excessive insertion of the blade. For example, if a 10 Fr red rubber feeding tube will be used (approximate outer diameter of tube is 6 mm), then the scalpel blade should be held such that the maximum exposed width of the blade (at the fingertips) is 6 or 7 mm.
• The tube is inserted into the abdomen. Tube insertion is facilitated by grasping the tip in the lower jaw of an alligator forceps, closing the forceps, and advancing tube and forceps through the hole. Mosquito forceps are an acceptable alternative. Often the hole in the skin and the hole in the body wall are not exactly aligned because of imperceptible shifting of the tissue planes. Blunt probing with the tube and forceps may be necessary to find the hole in the abdominal wall. If excessive pressure is required, the incision may need to be enlarged using the #11 scalpel blade.
• Any sign of discomfort on the animal’s part is an indication for additional lidocaine infiltration at and around the insertion site.
• Once the tube is inserted appropriately (a release of pressure may be apparent as the tube pierces the peritoneum and initiates voluminous flow of ascites), it is advanced until it protrudes from the abdominal wall by only 1-2 inches (several cm).
• The tube is sutured in place, using 2-0 or 3-0 nylon, with both a circumferential purse string and a transfixation (suture through the tube) ligature.
• If a rapid flow of fluid occurs, a clamp or partially closed three-way stopcock (usually requiring a “Christmas tree” type of adapter to fit most red rubber feeding tubes) can be used for moderating the rate of flow.
• Complete drainage is possible in minutes (often 15-20 minutes) or 2-6 hours (animal is placed in a cage with a grated floor to allow drainage; towels are placed on top of the grate to prevent a clamp/stopcock from becoming caught in the grate).
• Caution: An Elizabethan collar is essential for preventing the animal from chewing at and transecting the tube.
• The system may be closed (drainage bag) or open; if open, as is done most commonly, the animal must be monitored for ongoing drainage, and the tube should be removed immediately when flow ceases to reduce the risk of ascending infection.
• When drainage has ended, the animal is again restrained in lateral recumbency, and the nylon ligatures are cut. The tube is removed, taking care not to withdraw omentum. The skin incision may be dried with a sterile gauze, and tissue glue may be applied to close it. If the incision is >5 mm, a skin suture or staple may be placed.
POSTPROCEDURE
• Weigh the animal; record weight of lost fluid (for future reference and also to know accurate lean body weight for medication dosages).
• Dripping of ascitic fluid from incision is common despite tissue glue and generally resolves in minutes to hours. If it is persistent, a skin suture or staple may be necessary.
Abdominocentesis
CONTRAINDICATIONS
• Abdominal enlargement due to mass or organomegaly, without ascites, because of risk of organ damage without benefit of fluid retrieval for analysis
EQUIPMENT, ANESTHESIA
• Procedure is done with manual restraint only and without anesthesia (local or general), except in fractious or excited animals.
• Note: Pressure of large-volume ascites on the diaphragm may distress animals during restraint. In such cases, sedation should not be administered, but rather the procedure may be performed with the animal in lateral recumbency or in standing position.
• Needles (1-5; 2 inches long; 20-, 22-, or 25-gauge based on animal size and body wall thickness); a 22-gauge needle is generally preferred.
PREPARATION: IMPORTANT CHECKPOINTS
• Assess for risk of bleeding disorder if indicated overtly (e.g., epistaxis, hematuria, melena, petechiae/ecchymoses) or suspected via primary diagnosis (e.g., hepatopathy).
POSSIBLE COMPLICATIONS AND COMMON ERRORS TO AVOID
• “Dry tap,” no fluid withdrawn: insufficient abdominal fluid (check with ultrasound); fluid is too viscous or particulate (change needle and syringe because clot may be in needle; check fluid appearance with ultrasound); wrong area was sampled (change animal’s posture—e.g., try with animal standing—or site of centesis).
• Centesis not representative of underlying process: assess for extraabdominal causes of ascites such as cardiovascular, hypoalbuminemia, and vasculitis as indicated, and pursue primary intraabdominal causes further, beginning with abdominal ultrasound. If negative, consider nonexfoliating intraabdominal disease.
PROCEDURE
• Place animal in dorsal recumbency or lateral recumbency. If dyspnea is present, lateral recumbency is preferable. Very small, dyspneic animals (cats, toy-breed dogs) that are cooperative may be restrained in an upright sitting position (“begging” posture) in a technician’s arms; muzzling is recommended owing to proximity to technician.
• Aseptically scrub and prepare the clipped area, which typically will be on the midline just cranial or just caudal to the umbilicus. Position adjusted according to any known risk (e.g., cavitated abdominal mass in that location).
• Connect needle to syringe and advance needle through skin and body wall, directing the needle cranially and dorsally into the abdomen. The needle is meant to pass through the linea alba/body wall slightly obliquely, such that the tract left when the needle is removed can close on itself. The obliquity comes from the dorsocranial orientation of the needle on the midline instead of direct dorsal orientation.
• Once tip of needle is just under the skin, pull back on syringe plunger intermittently or continuously and observe for fluid “flashback” into syringe.
• Once fluid flow is established, the desired amount of fluid can be aspirated, and then the needle is withdrawn from the abdomen smoothly and quickly.
• Immediate tests that can be performed include:
○ Visual inspection for turbidity and color. Note: Hemorrhagic samples with hematocrit > 5% appear as red as blood.
○ Odor; foul smell commonly associated with anaerobic infections. Note: Be aware of the possibility of airborne zoonoses if applicable (e.g., systemic fungal, other), and avoid assessing odor in such cases.
• The fluid should be fractionated into two tubes (EDTA [lavender-top tube], and sterile glass [red-top tube]), and a sample should also be saved on a sterile swab for bacterial culture. The two tubes are submitted for standard fluid analysis including cytologic evaluation. The bacterial swab can be submitted for culture immediately if bacterial infection is suspected clinically, or it can be saved for later submission in case cytologic results indicate the possibility of bacterial infection.
Acupuncture 
OVERVIEW AND GOAL
• Acupuncture may be used in concert with other methods of integrative pain management for the control of acute or chronic pain.
• Most clinicians report that acupuncture either resolves the clinical problem or serves well as a maintenance therapy for a lifelong condition in 80% of cases.
• There are several indications for the use of acupuncture as an adjunct treatment for specific visceral organ diseases.
• The many different techniques used for stimulating an acupuncture point may be a source of confusion (e.g. heat, ultrasound, laser, or implant). Each method has its purpose and particular virtues in a given clinical setting.
• There are several neurophysiologic mechanisms which describe acupuncture effects. Spinal gates, central nervous system (CNS) endorphin release, and viscerocutaneous reflex arcs are a few of these.
• The teaching and clinical applications of acupuncture may be based on any of several different methodologies. The principle systems are known as traditional Chinese medicine (TCM); the eight principles;and medical, empirical,and French energetic acupuncture.The essential differences between them are matters of history and clinical philosophies. Each system uses the same needles and inserts them into the same acupuncture points. Differences of clinical outcome between these methodologies are difficult to measure and appear to be slight.
CONTRAINDICATIONS
• Pregnancy and tumors are often cited as relative contraindications. Use of acupuncture therapy in patients with these conditions should be reserved for clinicians with advanced knowledge of safe protocols.
ANTICIPATED TIME
• Particular problems may be resolved following a single treatment, but most will need from 3-8 treatments.
PREPARATION: IMPORTANT CHECKPOINTS
• Acupuncture may be performed on the sedated or anesthetized patient, but this is seldom indicated and may cause more problems than it prevents.
• Acupuncture will almost always be less difficult and more acceptable when performed without physical restraint.
• Medium- and large-breed dogs typically will be more relaxed when treated on a low bench or on a blanket on the floor instead of the typical exam table.
POSSIBLE COMPLICATIONS AND COMMON ERRORS TO BE AVOIDED
• Clinically relevant complications of acupuncture in animals are rare, and patients do not require hospitalization.
• A stuck or “frozen” needle (resists removal) will often occur: this is easily resolved by further manipulation of the needle for a few more minutes.
• Immediately following the first treatment, patients may show a transient increase in disease signs of the condition being treated.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree