CHAPTER 7. Pregnancy: Physiology and Diagnosis
OBJECTIVES
While studying the information covered in this chapter, the reader should attempt to:
■ Acquire a working understanding of the physiologic events of pregnancy in the mare.
■ Acquire a working knowledge of the procedures used to diagnose pregnancy in the mare.
STUDY QUESTIONS
1. Discuss the following physiologic events of pregnancy in the mare:
a. Entrance of embryo into the uterus
b. Conceptus mobility throughout the uterus
c. Embryonic vesicle fixation
d. Maternal recognition of pregnancy
e. Endometrial cup formation
f. Supplementary corpus luteum (CL) formation
g. Placentation
2. Describe the hormonal events of pregnancy in the mare, paying particular attention to source and timing of progesterone production (i.e., primary CL of pregnancy, supplementary CL of pregnancy, placental progestogen production). Also include in your description the concentrations of estrogen and equine chorionic gonadotropin in the blood stream of the mare.
3. Discuss methods of pregnancy detection at various stages of gestation in the mare. Include advantages and disadvantages of each method.
a. Behavioral assessment
b. Progesterone assays
c. Vaginal speculum examination
d. Palpation per rectum
e. Transrectal ultrasound examination
f. Equine chorionic gonadotropin (eCG) detection
g. Serum estrone sulfate detection
h. Urinary estrone sulfate detection
EARLY EVENTS OF PREGNANCY
Maternal endocrine status during the first 14 days of pregnancy is similar to that of the nonpregnant mare in diestrus. If the mare is not pregnant, the endometrium releases prostaglandin-F 2α (PGF 2α) on approximately days 14 to 15 after ovulation, which causes regression of the corpus luteum and permits the mare to return to estrus. If the mare is pregnant, the corpus luteum does not undergo lysis on days 14 to 15 but persists and continues to secrete progesterone, which is responsible for pregnancy maintenance. The process whereby luteolysis is prevented by the presence of the conceptus is referred to as maternal recognition of pregnancy.
The embryo enters the uterine lumen approximately 6 days after ovulation. The early equine conceptus has been shown to be endocrinologically active, with the young embryo expressing enzymes for synthesis of prostaglandins E and F (PGE and PGF, respectively). The signal for activating this prostaglandin synthesis is still unknown. Idaho workers showed that the morula and early blastocyst secrete prostaglandin E (PGE), which plays a role in transport of the embryo from the oviduct into the uterus. Later in development, the early embryo secretes PGF, which is thought to stimulate myometrial contractions.
Unlike embryos of other domestic species, which typically remain in the uterine horn ipsilateral to the ovulatory ovary and elongate to facilitate maternal recognition of pregnancy, the equine embryonic vesicle remains spherical and is quite mobile after its descent into the uterus, migrating through both uterine horns and the uterine body several times each day. Mobility is maximal on day 11 or 12 and is maintained to approximately day 16. Movement of the embryonic vesicle is passive, dependent on uterine contractions that propel it through the uterine lumen. However, experiments have shown that simulated conceptuses move more slowly through the uterus than do viable conceptuses, which indicates that the embryo itself provides an active stimulus to uterine mobility.
Movement throughout the uterus is thought to be important for the conceptus to “ signal” the dam that pregnancy has occurred (because of the small trophoblastic surface of the equine conceptus compared with that of other species), thereby preventing luteolysis. The factor involved in maternal recognition of pregnancy has not been identified in the equine, but in vitro studies have shown that the conceptus secretes a low–molecular weight (≤10 kDa) protein that inhibits PGF 2α production by the endometrium.
Another unique feature of the equine embryo, described by Canadian workers, is the formation of a polysaccharide-rich capsule that forms between the trophectoderm and zona pellucida of the embryo within 48 hours of entering the uterus. The capsule continues to grow along with the embryonic vesicle until about day 18 and thereafter disappears by day 23 of pregnancy. The capsule is postulated to protect the conceptus from mechanical damage when it is propelled throughout the uterus during the mobility phase and may also be involved in protecting the conceptus from being recognized as immunologically foreign to the dam. The capsule undergoes rapid changes in protein composition at the time the conceptus becomes fixed within the uterus.
The corpus luteum, called the primary corpus luteum (CL) of pregnancy, can be visibly identified in the ovary for up to 180 to 220 days. The primary CL regresses at approximately the same stage of gestation as the supplementary corpora lutea. The critical time for maternal recognition of pregnancy, to prevent luteolysis of the primary CL and subsequent loss of the pregnancy, is thought to be 14 to 16 days after ovulation in the mare. Suppression of mobility of the conceptus through the uterus during days 10 to 16, or conditions that prevent the conceptus from migrating throughout the uterus (e.g., blocked uterine horn), will interfere with maternal recognition of pregnancy, resulting in failure to prevent endometrial production and release of PGF 2α, and the mare returns to estrus in spite of conceiving.
The embryonic vesicle becomes stationary by approximately day 16 after ovulation. This process is referred to as fixation and is apparently largely the result of restriction of the enlarging conceptus at the base of one uterine horn. The process of embryonic vesicle fixation should not be confused with the process of fetal-maternal attachment. Soon after the time of fixation, the cross-sectional shape of the embryonic vesicle typically changes from circular to triangular; this change in shape is identified by transrectal ultrasonographic examination. The change in vesicle shape is the result of thickening (hypertrophy) of the dorsal uterine wall (see Figures 5-30 and 5-31). The encroachment of the dorsal aspect of the endometrium onto the vesicle is thought to play a role in orienting the embryo proper to a ventral 6 o’clock position, opposite the mesometrial attachment (antimesometrial).
When the reproductive tract is under progesterone influence from the primary CL of pregnancy and estrogen influence from the developing conceptus (estrogen is produced by day 12), the uterus begins to develop a characteristic shape with marked tone that becomes readily apparent on palpation per rectum by day 16 to 18 after ovulation (Figure 7-1). Examination via palpation per rectum usually reveals suggestive, but not definitive, evidence of early pregnancy at this point because the conceptus may not yet be readily palpable. The conceptus does not begin attaching to the endometrium until day 40 to 45 of gestation, so the increased tone of early pregnancy is thought to help keep the embryo and developing membranes in close apposition with the endometrium during this time period to maximize nutrient transfer. The advent of ultrasonography has allowed considerable progress in pregnancy diagnostics in the mare because this technique enables detection of embryonic vesicles in the uterus as early as 9 to 10 days after ovulation. Pregnancy can typically be confirmed via palpation per rectum by most examiners at 25 to 35 days of gestation.
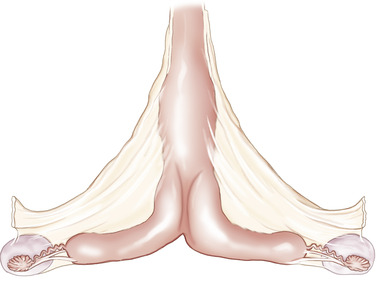
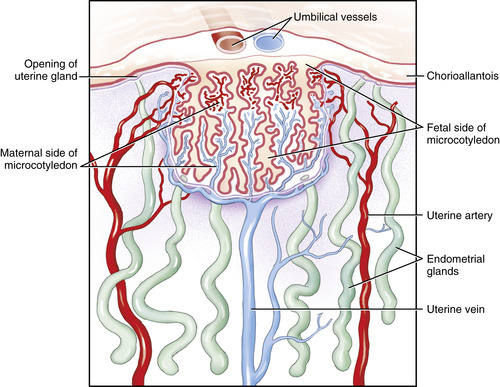
The process of fetal-maternal attachment is gradual. The vascularized trophoblast is closely associated with the uterine epithelium by day 25. Beginning interdigitation of trophoblastic microvilli and uterine epithelium has been described as occurring by days 38 to 40. Fetal macrovilli (which become the microcotyledons and are distinct from microvilli) begin appearing by day 45 as rudimentary structures and gradually develop until full placental attachment occurs in the form of well-developed microplacentomes (microcotyledons with maternal microcaruncles) by day 150 (Figure 7-2).
ENDOMETRIAL CUP FORMATION
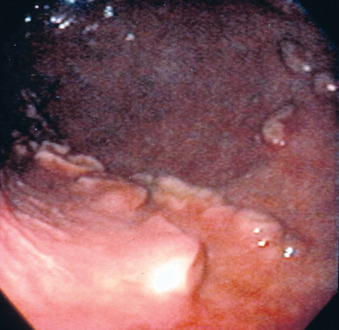
A unique feature of the equine placenta is the development of endometrial cups early in gestation. On about day 25 of gestation, a specialized annular band of the trophoblast undergoes cellular changes to form the chorionic girdle at the junction of the developing allantois and regressing yolk sac. These trophoblastic cells of the chorionic girdle invade the adjacent uterine epithelium on approximately day 38 of gestation, migrating into the underlying lamina propria to develop into the prominent decidua-like cells of the endometrial cups. The endometrial cups are identified as a circular or horseshoe arrangement of pale irregular outgrowths on the luminal surface of the gravid uterine horn (Figure 7-3). They attain maximal size at about day 70 of gestation and then begin to undergo degeneration and eventually are completely sloughed by approximately day 130 of gestation. The endometrial cups secrete a hormone known as equine chorionic gonadotropin (eCG), previously termed pregnant mare serum gonadotropin (PMSG). Equine chorionic gonadotropin is first detectable in maternal blood on days 35 to 42, rapidly increasing in concentration to peak levels on days 55 to 65; then concentrations decline slowly to low or nondetectable levels by days 100 to 150. This hormone is believed to assist in the formation of supplementary corpora lutea and is thought to also be a necessary stimulus for maintenance of the primary CL during approximately days 35 to 120. Progesterone output from the primary CL actually increases during days 35 to 40, before acquisition of significant progesterone production by the supplementary corpora lutea. Equine chorionic gonadotropin is also thought to play an important immunoregulatory role during pregnancy.
Endometrial cup formation is an important consideration in the management of early embryonic loss. If pregnancy loss occurs before the formation of the endometrial cups (days 36 to 40), the mare returns to estrus within a short period of time (less than 1 month). Because only the primary CL is present, a single injection of PGF 2α promotes CL regression and return to estrus. If pregnancy loss occurs after endometrial cup formation, eCG production continues and the mare may not return to estrus for 3 months, when the endometrial cups degenerate, thereby allowing supplementary corpora lutea to regress. In addition, a mare that loses a conceptus after days 36 to 40 of gestation remains positive for pregnancy according to eCG detection tests (e.g., Equi-Chek, Endocrine Technologies Inc, Newark, Calif.) for up to 3 months although the mare is no longer pregnant, because these tests assay for presence of circulating blood levels of eCG, not pregnancy itself. Of additional importance, repeated PGF2α injections may cause a mare that has aborted after 40 days of gestation to return to estrus by inducing regression of supplementary corpora lutea, but the estrus is seldom fertile as long as endometrial cups remain. Another aggravating problem when attempting to rebreed a mare that has endometrial cups remaining after abortion, even when the mare has been induced to return to estrus with repeated PGF 2α injections, is the tendency for follicles to fail to ovulate. Such mares have also been noted to ovulate smaller-than-normal follicles, luteinize follicles that do not ovulate, or form structures similar in appearance to hemorrhagic anovulatory follicles (Figure 7-4).
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree