CHAPTER 3. Manipulation of Estrus in the Mare
OBJECTIVES
While studying the information covered in this chapter, the reader should attempt to:
■ Understand potential advantages for manipulating estrus in broodmares.
■ Acquire a working understanding of the principles by which manipulation of the photoperiod advances the onset of the breeding season in mares.
■ Acquire a working knowledge of the rationale for, and efficacy of, hormone administration for hastening the onset of the breeding season in mares.
■ Acquire a working knowledge of the use of various hormones for manipulating estrus in the cyclic mare, including the first postpartum estrus.
STUDY QUESTIONS
1. Describe the physiologic changes that occur during transition from seasonal anestrus through onset of the breeding season in mares in the northern hemisphere.
2. Define the following terms:
a. Seasonal (winter) anestrus
b. Transitional estrus
c. Ovulatory estrus
d. Artificial photoperiod
3. List guidelines to be followed when designing an artificial lighting program for broodmares in the Northern Hemisphere.
4. Discuss the rationale for use of progestogen/progesterone to hasten (synchronize) the onset of ovulatory estrus in mares.
5. Outline a program (and include expected responses) for treating mares with altrenogest to:
a. Hasten (synchronize) the onset of ovulatory estrus early in the breeding season.
b. Synchronize estrus in cyclic mares.
6. Outline a program (and include expected responses) for treating mares with progesterone and estradiol-17β plus prostaglandin-F 2α (PGF 2α) to synchronize estrus and ovulation in cyclic mares.
7. Outline a program (and include expected responses) for use of human chorionic gonadotropin (hCG) (Chorulon, Intervet/Shering-Plough Animal Health, Whitehouse Station, N.J.) or deslorelin (Ovuplant, Fort Dodge Laboratories, Kalamazoo, MI) to induce ovulation in mares:
a. In late transitional estrus.
b. That have regular estrous cycles but are to be bred at a precise time.
8. Outline a program (and include expected responses) for use of PGF 2α to:
a. Induce estrus in a mare with a functional corpus luteum.
b. Synchronize estrus in a group of cyclic mares.
c. Induce earlier estrus in mares after foal-heat ovulation.
Registry-derived time constraints, in conjunction with a seasonal breeding pattern, result in use of a narrowly confined breeding season to produce most equine offspring. In the operational breeding season (mid February through the first week in July), the veterinarian is often called on to assist in breeding mares early in this time period to facilitate birth of foals as soon after January 1 as possible. In addition, to maximally utilize genetically superior, popular stallions, veterinary management schemes have been developed to limit the number of services per estrus to the minimum necessary to establish pregnancy. The ascribed goal is one pregnancy per service per mare. To these ends, artificial lighting programs and hormone administration are used to (1) hasten the onset of the breeding season; (2) induce ovulation in cyclic mares being bred; (3) synchronize estrus and ovulation in individual mares or groups of mares; and (4) increase the opportunity for establishment of pregnancy in foaling mares bred early in the postpartum period. Table 3-1 presents a summary of hormones commonly used in broodmare practice. This table also lists hormones used for purposes other than manipulation of estrus (e.g., induced abortion, induced parturition), which are discussed in later chapters.
| Compound | Actions | Indications | Product | Source | Dosage | Comments |
|---|---|---|---|---|---|---|
| GnRH (gonadotropin- releasing hormone) | Release of FSH and LH from anterior pituitary gland | Stimulation of follicular growth Ovulation induction (?) | Cystorelin (synthesized native GnRH) Fertagyl (Gonadorelin) | Ceva Intervet/Schering-Plough | Suggested dosages of 500 μg twice daily or 250 μg 4 times daily; IM or SQ | Not licensed for use in horses. Long/term exogenous GnRH stimulates follicular growth in seasonally anestrous mares but may not be cost effective. Mares treated late in the transition period (larger follicles) are more likely to respond with follicular growth, ovulation and formation of a normal CL, and in less time, than mares with static ovaries (winter anestrus) or in early transition (smaller follicles). |
| Deslorelin (GnRH analog) | Same, longer action, more potent | Ovulation induction | Ovuplant (deslorelin implant) | Fort Dodge | Sustained release SQ implant containing 2.1 mg deslorelin acetate | Administer 1 implant once estral mare has a follicle ≥30 mm (35 mm may be better) in diameter; more than 80% of mares ovulate within 48 hours. Extended interovulatory intervals occur in some mares that fail to become pregnant due to gonadotroph downregulation. Placing in vulvar mucosa allows easy removal after ovulation, and will avoid subsequent follicular suppression. |
| Bio Release Deslorelin Injectable Deselorelin injection | BET Pharm Compounding pharmacies | Sustained release IM injection containing 1.5 mg deslorelin Use as above. | Use as above; no evidence of follicular suppression following its use, so may not result in downregulation of pituitary gonadotrophs. Use as above. Dosages of 63 μg twice daily can hasten onset of first ovulation of year according to Canadian workers. | |||
| Oxytocin | Myometrial contractions | Parturition induction | Oxytocin | Several sources | 40-100 IU IM (bolus injection or slow IV drip) or 5-15 IU IV or IM q 15 min until 2nd stage of parturition | Can cause premature placental separation. Do not use in dystocia until fetal position/posture is corrected. |
| See previous page | Uterine evacuation (can be done in conjunction with uterine lavage) | 20-40 IU IV | Ensure that cervix is patent before use. | |||
| Expulsion of retained placenta | 10-20 IU IV or IM, can repeat at 2-4 hr intervals as needed | May cause abdominal cramps or, rarely, uterine prolapse. | ||||
| Acceleration of uterine involution after dystocia | 10-20 IU IM q 8-24 hr | May cause abdominal cramps or, rarely, uterine prolapse. Will not accelerate uterine involution in normal foaling mares. | ||||
| Contraction of myoepithelial cells in mammary gland | Milk letdown | 20 IU IV or IM | Generally unsuccessful in mares that are extremely nervous or suffer from agalactia. | |||
| Estrogens | Expression of estrus Maturation of reproductive tract and mammary glands Increased uterine circulation Uterine contraction | Expression of estrus in “jump” mare (for semen collection) | Estradiol cypionate (ECP) | Upjohn | 1-2 mg IM | Only effective in the absence of progesterone. Do not use in mares to be bred. Not approved for use in the horse. |
| Cervical relaxation Resistance to uterine infection | Treatment of infectious endometritis | Estradiol-17β | Compounding pharmacy | 10 mg/day IM for 1-3 days | Usefulness questionable. | |
| Suppression of follicular growth to improve synchrony of ovarian response | Synchron-ization of ovulation | Estradiol-17β | 10 mg/day IM for 10 days | Used in conjunction with progesterone in oil (150 mg/day for 10 days) and prostaglandin (on day 10 only). | ||
| Postponement of “foal heat” | Estradiol-17β | 10 mg/day IM for 1-10 days | Use in conjunction with progesterone in oil (150 mg/day). | |||
| Progestogens | Inhibition of LH release Suppression of estrus Reduce myometrial excitability Increase uterine tone Endometrial gland growth | Shorten duration of transitional season | Progesterone in oil | Compounding pharmacy | 150 mg/day IM for 10-15 days | Can use in conjunction with estradiol-17β (10 mg/day IM) for 10-15 days and prostaglandin (on last day of treatment). Effective only in mares during late transitional phase |
| Altrenogest (Regu-Mate) | Intervet/Shering-Plough | 0.044 mg/kg/day orally for 10-15 days | Effective only in mares during late transitional phase. Administering prostaglandin on the last day of treatment may improve results if the mare(s) ovulated during treatment. | |||
| Cervical closure | Suppression of estrus | Progesterone in oil | 150 mg/day IM | May take 2-3 days for mare to go out of behavioral estrus. | ||
| Mammary gland development | Altrenogest (Regu-Mate) | 0.044 mg/kg/day orally | Does not appear to affect subsequent fertility when given for 30-60 consecutive days. | |||
| Synchronization of ovulation | Progesterone in oil Altrenogest (Regu-Mate) | 150 mg/day IM for 10 days 0.044 mg/kg/day orally for 10-15 days | Use in comjunction with estradiol-17β (10 mg/day) and prostaglandins (on day 10 only) for best results.Administer prostaglandin on last day of treatment for best results. | |||
| Maintenance of pregnancy in ovariectomized recipient mares for embryo transfer | Progesterone in oil | 300 mg/day IM | Begin injections 5 days before embryo transfer and continue for first 100-120 days of pregnancy. | |||
| Pregnancy maintenance in habitually aborting intact mares. | Progesterone in oil Altrenogest (Regu-Mate) | 150-300 mg/day IM 0.044 mg/kg/day orally | Efficacy is controversial. Should be given until at least day 100-120 of pregnancy, regardless of whether giving altrenogest or progesterone in oil; may be some risk of fetal mummification if fetus dies and is not expelled. For late-aborting mares, should be given until just before expected parturition. | |||
| See previous page | Synchronize estrus | CIDR (controlled intravaginal drug release device) | Contains 1.9 g progesterone; release profile 14 days | Place in vagina for 12-14 days; alternatively, administer 8 days and give PGF 2α on day 8 at removal. | Available in North America for use in cattle; not approved for use in horses. Usually results in mild vaginal discharge that does not affect fertility; use aseptic technique at insertion and at removal to minimize chances of ascending infection. Expect efficacy similar to that of other progesterone/progestogen compounds. | |
| hCG (human chorionic gonadotropin) | Support of the CL of pregnancy in women. Has LH activity in the horse. | Ovulation induction | hCG (Chorulon) | Intervet/Shering-Plough, other sources | 1500-3500 IU given IM or IV | Administer hCG once a ≥35-mm follicle is detected on the ovary during estrus. Ovulation usually occurs within 36-48 hr after hCG injection. |
| Hasten ovulation in transitional mares | hCG | Same as above | 1500-3500 IU given IM or IV | Administer hCG once a 40-mm follicle is detected. Not approved for IV use in the horse, but is most commonly given IV. | ||
| Prostaglandins (PGF 2α) | Regression of CL Myometrial contractility Influence on numerous body functions | Shorten interovulatory period (“short-cycle” or induce estrus in cycling mares) | Dinoprost trometha-mine (Lutalyse) | Upjohn | 10 mg/1000 lb IM | Give at least 5-6 days after ovulation is detected. Microdose is 0.5 mg once or twice at 24-hour interval; decline in progesterone is slower, but side-effects are reduced (may not be as effective as microdose of cloprostenol). |
| Cloprostenol (Estrumate) | Miles | 250 μg/1000 IM | Not approved for use in horses in the U.S., but is commonly used. Microdose is 0.25 μg once or twice at 24-hour interval; decline in progesterone is slower, but side effects are reduced. | |||
| Synchronization of estrus | Same as above | Same as above | Same as above | Give two injections, 14 days apart. Effective only in mares with normal estrous cycles. | ||
| Treatment of persistent luteal function | Same as above | Same as above | Same as above | Synthetics and “natural” prostaglandin (dinoprost) are equally effective in causing CL regression, but synthetics have advantage of fewer side effects, such as sweating and abnormal cramping. | ||
| See previous page | Shorten interval to 2nd postpartum estrus | Same as above | Same as above | Same as above | Give prostaglandin 5-6 days after “foal heat” ovulation. | |
| Induction of abortion | Same as above | Same as above | Same as above | Single injection of prostaglandin is sufficient if given by 35 days of pregnancy. Multiple injections of prostaglandin are required once supplementary corpora lutea are formed (beyond 36-40 days of pregnancy). Prostaglandins may be ineffective beyond 4 months of gestation. | ||
| Acceleration of uterine involution | Same as above | Miles | 1 dose twice daily for first 5-10 days after foaling | Of questionable value in normal foaling mares. | ||
| Uterine clearance of fluid | Cloprostenal | Miles | 250 μg IM | For uterine clearance of retained fluid: cloprostenol maintains uterine contractions for 2-4 hours. Should probably not be given after ovulation, as may adversely affect CL function. | ||
| Induction of parturition | Cloprostenal | Miles | 250 μg IM twice at 2 hr intervals | Studies (limited) suggest various prostaglandin analogues (not dinoprost) can be safely used to induce parturition; however, oxytocin remains the drug of choice. | ||
| Ergonovine | Smooth muscle contractions; also aids contraction of smooth muscle in vascular walls to control hemorrhage | Control of postpartum hemorrhage | Ergonovine maleate or methylergonovine maleate | Several sources | 1-3 mg IM/1000 lb mare | Produces strong uterine contractions within 10-20 min after injection. Contractions may continue for up to 2-4 hr. Contractions are continuous rather than rhythmic, oxytocin-like contractions. Also aids in controlling certain types of uterine hemorrhage (i.e., uterine rupture; endometrial lacerations); ecbolics should not be used if uterine artery rupture is suspected. |
| Hasten uterine involution | Same as above | Same as above | Same as above | Unproven. | ||
| Bromocriptine | Inhibits prolactin secretion | Spurious lactation in non-gravid mares | Bromocrip-tine mesylate | Several sources | 30-60 mg/1000 lb mare orally once daily for 3-7 days | Human product; not licensed for use in horses. |
| Domperidone | Dopamine D2 receptor antagonist Increases prolactin secretion | Agalactia | Equidone (domperidone paste) | Equitox, Center for Applied Technology | 1.1 mg/kg orally once daily | Maintains elevated prolactin concentration for 7-9 hours. Increases prolactin to supraphysiologic levels within 7 days. Should be given daily to postpartum agalactic/hypogalactic mares until full milk production is achieved. Foal may need additional nutritional supplementation. Can be started 10-15 days prior to expected foaling in mares grazing endophyte-infested fescue pastures in attempt to decrease parturient problems and agalactia. |
| Anestrus | Same as above | Same as above | Same as above | Has been proposed as a treatment for anestrus, to stimulate ovarian follicular activity and advance the first ovulation of the year. Dopamine is thought to inhibit gonadotropin production; therefore, dopamine antagonism is the rationale for its use. Alternatively, it may act directly at the ovary. 2-4 weeks may be required to increase LH levels, so begin treatment in late December or early January. Contradictory results obtained in various studies. | ||
| Sulpiride | Dopamine D-2 receptor antagonist | Anestrus | No commercial preparation | Compounding pharmacy | 100-200 mg/454 kg body wt, once daily | Rationale is as proposed for domperidone. May hasten response to lighting program if give 14-16 hr light for 2 weeks, then begin daily sulpiride injections; if respond (60-80%), usually do so by ovulating within 15 days of 1st sulpiride injection (sometimes by 8-11 days). Whether should induce ovulation with hCG or deslorelin remains unstudied, but an ovulation-inducing drug is usually given. Concurrent estrogen administration may further increase prolactin and thus improve response. |
| eFSH | Stimulate follicular growth | Induction of multiple ovulations | Purified equine FSH | Bioniche Animal Health | 12.5 mg eFSH twice daily for 3-5 days | CSU protocol—begin eFSH 5-7 days after ovulation. Administer PGF 2α on 2nd day of eFSH treatment, and continue eFSH until follicles reach 32-35 mm in diameter. Allow 36 h of no treatments, then administer hCG to induce ovulations. Attempt embryo recovery 7-8 days after ovulation(s). May no longer be available. |
| Hasten onset of ovulatory estrus in transition period | 12.5 mg injected IM twice daily once ≥25-mm follicle(s) are present; stop treatment once follicle reaches 35 mm and administer hCG IV | Most ovulations (in responding mares) occur between 5-10 days after beginning treatment; 20-30% failures reported. Caution—can induce multiple ovulations which may result in multiple pregnancies. | ||||
| reLH | Stimulate follicular maturation and ovulation | Induce ovulation | Recombinant equine LH | Not yet commercially available | 750-1500 μg injected IV | Administration to estrual mares once mature follicle reaches 35 mm in diameter results in ovulation response similar to hCG (80-90% ovulations within 2 days). |
ARTIFICIAL LIGHTING
The physiologic breeding season can be successfully manipulated to fit into the operational breeding season by artificially increasing the photoperiod. The minimal length of light exposure necessary has not been critically established, but field experience indicates that 14 to 16 hours of light stimulus (artificial plus natural) per day is adequate. A recent French study provided evidence that high light intensities may not require as many days of lighting to induce cyclicity in anestrous mares and that low light intensities may not be as efficacious in stimulating desired responses. Nevertheless, because lighting programs have traditionally been thought to require a minimum of 8 to 10 weeks for response, mares in the northern hemisphere are exposed to the lighting system by December 1 to establish normal cyclic activity by mid-February. Various methods of light administration have been used successfully, the most common of which are (1) use of a light source that is held steady at 14 to 16 hours/day throughout the entire stimulation period; or alternatively, (2) increasing light by small increments (similar to that which occurs naturally), usually by adding 30 minutes of daily light stimulation at weekly intervals until 14 to 16 hours of light exposure is achieved (e.g., 10 hours December 1, 10.5 hours December 8, and so on). After summarizing results of various lighting programs, Sharp et al. (1993) suggested best results are obtained when the supplemental light is either added to the end of the day or split and added to both the beginning and the end of the day, instead of adding the supplemental light only at the beginning of the day. Palmer and coworkers (1982) have described a technique for providing a 1-hour pulse of light 18.5 hours after the onset of daylight. This dark-phase light pulse has reportedly resulted in resumption of reproductive cyclicity similar to that observed with the more traditional lighting techniques. The efficacy of this lighting technique should probably be studied further before being applied to commercial operations.
For individual stall-lighting systems, Kenney et al. (1975) recommended the mare should be within 7 to 8 feet of a 200-watt incandescent light bulb to provide adequate light exposure and the stall should have sufficient window space to permit the same exposure during daylight. Minimal light intensities have not been adequately determined. Sharp et al. (1993) recommend a minimum intensity of 10 foot-candles at mare eye level. The presence of shadows can prevent achievement of desired results, so care should be taken to eliminate them. Paddock lighting systems are also successful if light exposure is sufficient in all areas of the paddock. Guidelines for ensuring adequate light exposure in paddock lighting programs have been reviewed by Ginther (1992). A practical method for measuring light intensity has been described by Sharp et al. (1993) wherein the American Standards Association (ASA) reading of a 35-mm single-lens reflex camera is set to 400 and the shutter speed to 1/4 second. The bottom of a Styrofoam cup is cut off, and the cup is fitted over the lens to gather diffused light. If the aperture reading is f4, light intensity is 10 foot-candles.
With artificial lighting systems, a widely held belief is that pregnant mares should also be exposed to lights because early-foaling mares that are not exposed to lights are thought to be at risk for entering seasonal anestrus. Kentucky workers recently showed that no consistency exists regarding when mares enter anestrus (e.g., an individual mare may enter seasonal anestrus in November of one year and in December or January of the next year; and an individual mare that cycled throughout one year may enter seasonal anestrus the next year), so provision of artificial lighting to all mares to be bred on the farm may be critical if consistent responses to lighting are to be expected.
DOPAMINE D2 ANTAGONISTS
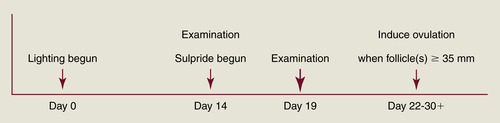
Although the mechanism of ovarian stimulation remains unclear, evidence shows that inhibition of dopamine activity in mares in late anestrus can shorten the interval to first ovulation of the year. Cornell workers have postulated that dopamine exerts a tonic inhibition of reproductive activity during the anovulatory season of the mare. Dopamine may mediate its effects through prolactin (PRL) secretion (PRL increases during the ovulatory season in relation to increasing day length), which could in turn stimulate expression of ovarian gonadotropin receptors. D2 receptor antagonists (sulpiride, domperidone) have been used to stimulate PRL secretion and are of particular use in the treatment of some forms of agalactia. D2 receptor antagonists have also been investigated for their potential to advance the ovulatory season in mares. Their success when used by themselves has been equivocal except in mares in mid to late transition. For example, Cornell University workers administered 200 mg sulpride once daily to anestrous mares beginning February 5 and were able to advance the time of the first ovulation of the year by more than a month (compared with untreated control mares), but mean treatment length was 41 days. However, recent results from the use of D2 receptor antagonists in combination with a lighting program have been encouraging. Duchamp and Daels (2002) reported that subjecting anestrous mares to 14.5 hours of light on January 10, with administration of sulpiride (1 mg/kg intramuscularly [IM] twice daily) beginning 2 weeks later, resulted in advancement of the first ovulation of the season in 86% of mares (17 days earlier than lighted mares not given sulpiride). One of the authors has used this program for noncyclic race-trained maiden mares arriving at the breeding farm in February or March (Figure 3-1). Mares are immediately placed on an artificial lighting program and are examined for ovarian activity 2 weeks later. If follicles 15 mm in diameter or more are present at this time, twice-daily intramuscular injections of sulpiride (200 mg) are begun. Beginning 5 days later, mares are examined every other day until a follicle achieves 30 mm in diameter. Examinations are daily thereafter, and once the dominant follicle reaches at least 35 mm in diameter and endometrial edema is present, an ovulation-inducing drug (hCG or deslorelin) is given and the mare is bred. For a group of 16 maiden mares treated in this manner during February and March 2006, 10 responded (64% response rate) to treatment and ovulated 13.4 ± 4.2 days (range, 8 to 22 days) after beginning sulpiride administration (treatment was discontinued in the six mares that showed no significant follicular growth after at least 10 days of treatment). Pregnancy rate was 50% (5 of 10) in the mares that responded to treatment, and all of the mares that did not become pregnant continued to have regular estrous cycles thereafter. Of the six mares that did not respond to sulpiride treatment, ovulation did not occur for 2 to 8 weeks.
SHORTENING THE DURATION OF THE LATE TRANSITION PERIOD WITH ADMINISTRATION OF PROGESTOGENS
Rationale for the use of progestogen/progesterone treatment for hastening the onset of ovulatory estrus is based on insufficient storage or release of luteinizing hormone (LH) from the pituitary in traditional estrus to promote maturation and ovulation of a dominant follicle. Progestogen treatment, which generally suppresses LH release during administration, has been postulated to provide for storage and subsequent release of sufficient LH to induce follicular maturation and ovulation once progestogen supplementation ceases. However, Colorado workers have questioned this hypothesis because they recently showed no greater release of LH in response to gonadotropin-releasing hormone (GnRH) (1 mg, pharmacologic dose) administration, and no shortening in mean interval to first ovulation, in six progestogen-treated transitional mares. These workers proposed the main advantage to progestogen treatment of late-transition mares is to synchronize the impending ovulation, thereby facilitating breeding at an opportune and predictable time. Nevertheless, variations of progestogen treatment of mares in transitional estrus remain commonplace in the equine industry.
Progesterone in oil (150 mg each day, IM) or altrenogest (0.044 mg/kg each day, orally [PO]) for 10 to 15 days is a common treatment regime for this purpose. Initial experiments with altrenogest, a synthetic progestogen (Regumate, Intervet/Shering-Plough Animal Health, Whitehouse Station, N.J.) tested efficacy of treatment in mares in early transition (<20-mm diameter follicles) compared with mares treated in late transition (>20-mm diameter follicles) and revealed that mares in early transition did not respond favorably to altrenogest treatment. For best results, current recommendations for the use of progestogen/progesterone are to first examine the mare’s ovaries via palpation or ultrasound per rectum to ensure multiple follicles 25 mm or more in diameter are present before instituting therapy. If mares are in early transitional estrus (i.e., only smaller follicles are present), they are unlikely to respond. Although progestogen/progesterone administration is expensive and time-consuming, best results may be achieved by longer (e.g., 2 weeks) durations of treatment, perhaps because of greater storage and subsequent release of LH that occurs as day length increases. Interval to estrus is somewhat variable after cessation of progestogen administration but averages 4 to 7 days, with ovulation usually occurring 7 to 12 days later.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree