Understanding the biology and genetic basis of cancer is imperative to improving our ability to provide effective treatments. This is a rapidly expanding field of knowledge and the purpose of this chapter is to introduce you to the existing level of understanding and the principles on which current and future treatments are based. Those readers interested in the basic science are referred to more advanced texts.
Tumour development and growth
Cancer is a genetic disease involving damage to the DNA leading to uncontrolled cellular growth. We know that there are a number of environmental factors and chemicals that can lead to the development of cancer (Table 2.1), including ultraviolet light (squamous cell carcinoma), aflatoxins (liver cancer) and viruses (leukaemia and lymphoma). To be carcinogenic these agents must cause damage at the genetic level.
| FeLV, feline leukaemia virus; FeSV, feline sarcoma virus; SCC, squamous cell carcinoma. | ||
| Agent | Neoplasm | Species |
|---|---|---|
| Cyclophosphamide | Bladder transitional cell carcinoma | Dog |
| Oestrogen | Mammary carcinoma | Dog |
| Testosterone | Perianal adenomas | Dog |
| Air pollution | Tonsillar SCC | Dog |
| Fracture/implant | Osteosarcoma | Dog |
| Ocular trauma | Intraocular sarcomas | Cat |
| Spirocerca lupi | Oesophageal sarcoma | Dog |
| External beam radiation | Osteosarcoma | Dog |
| UV radiation | Cutaneous SCC Cutaneous haemangioma/sarcoma | Dog, cat Dog |
| Papilloma virus | Oral SCC (papillary variant) | Young dogs |
| FeLV | Leukaemia/lymphoma | Cats |
| FeSV + FeLV | Fibrosarcomas | Young cats |
The two-step theory of tumour development
Current belief favours a two-step theory of tumour development. The first step involves exposure of the cell to the carcinogen, known as the ‘initiator’, and results in permanent alteration of the DNA. A long lag period may then occur and it can be months or years before the second step, known as promotion, allows the transformed cells to progress into a state of uncontrolled growth. The ‘promoter’ may be the same agent as the initiator, or may be a second agent including normal growth promoters or hormones. Once the initial events have taken place at the level of the DNA, changes in expression of regulatory genes lead to unrestricted growth and oncogenesis.
What are the characteristics of malignancy?
Rapid cell division is a characteristic of malignancy and growth occurs because the normal regulatory apparatus of the cells is defective – in other words, there is a breakdown in homeostasis at the molecular level. Tumour cells are further characterized by their independence from external mitogenic stimuli allowing sustained growth and by their ability to avoid anti-growth signals that would normally lead to terminal differentiation and the post-mitotic stage. Mechanistically this relies on the activation of cellular oncogenes (Table 2.2).
| For a more detailed list of oncogenes and their products, see Calvo et al (2005). | ||
| Class | Oncogene | Product |
|---|---|---|
| Class I: Growth factors | sis | Form of platelet-derived growth factor |
| Class II: Growth factor receptors | erb-b | Epidermal growth factor receptor |
| Class III: Intracellular transducers | Protein tyrosine kinases, e.g. met | Protein kinases that phosphorylate tyrosine residues |
| Protein serine-threonine kinases, e.g. mos | Protein kinases that phosphorylate serine or threonine | |
| Ras proteins, e.g. N-ras | Guanine nucleotide-binding protein with GTPase activity | |
| Class IV: Nuclear transcription factors | myc | Regulation of transcription |
For a tumour to establish itself it must rapidly develop a blood supply and to do that angiogenesis is required (Kerbel 2008). The ability of a growing tumour to induce angiogenesis is essential for its sustainability. The actual process is complex and involves factors produced by both host and tumour. Angiogenesis is maintained by a number of positive and negative signals that include soluble mediators and their receptors (integrins), and adhesion molecules that are responsible for cell and matrix interactions (Moschos et al 2007). Angiogenic factors include vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF).
Before a tumour can be detected clinically it must have reached a certain size. For superficial tumours, this means that approximately 109 cells are present and the tumour has undergone up to 30 doublings. The growth fraction of a tumour is not constant but decreases exponentially with time. If a tumour becomes very large there may be a deceleration in growth because of reducing oxygenation and nutrition within the tumour, leading to senescence or cell death and necrosis. Large tumours with a small growth fraction are resistant to modalities such as radiotherapy and chemotherapy that require cells to be actively dividing.
A characteristic of cancer cells is immortality. In part this feature resides in the production of the enzyme telomerase. Telomeres are the terminal ends of the chromosome and in normal cells these are progressively difficult to replicate with progressive cell divisions (Raynaud et al 2008). In normal cells the telomeres are so short that eventually the cell is made senescent. Tumour cells, however, have the capability of maintaining their telomeres by the production of telomerase. Telomerase is the underlying cause of immortality in cancer cells and is therefore a common marker of malignancy and potentially a therapeutic target (Harley 2008).
The goal of cancer treatment is cure. However, that goal is often not achieved because of the ability of malignant cells to metastasize.
What is metastasis?
Metastasis is the capability of malignant tumours to spread to distant sites and there develop into new tumours. It is combating the capability of cancer cells to metastasize that remains the most challenging aspect of cancer therapy. Primary tumours can, in the majority of cases, be controlled by surgery, radiotherapy or chemotherapy, or a combination of these modalities, especially when the tumour is detected early (good physical examination and prompt diagnostics) and treated appropriately. Unfortunately, even with early removal of the primary tumour, it is the ability of cancer cells to spread throughout the body, a process that commences as soon as the tumour has established access to the lymphatics and blood supply, that usually leads to treatment failure and remains the biggest challenge in the management of patients with cancer. Metastatic spread can occur early in the development of a malignancy, even before the tumour is detectable by current methods. The process by which cancer cells spread to secondary sites involves a number of steps as well as an intimate interaction between host and tumour, and is described as the metastatic cascade (Figure 2.1).
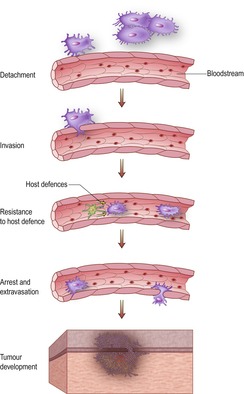 |
| Figure 2.1 |
The metastatic cascade (for further details see Hill 1992)
Detachment and invasion
At the beginning of the metastatic process cells have to be able to mobilize themselves and break down normal tissue barriers to enable them to gain access to small blood vessels. This requires the production and release of proteolytic enzymes, growth factors, cytokines, etc. The cancer cells themselves produce some of these ‘mediators of invasion’, whereas others are produced by normal tissue components stimulated by the neoplastic cells.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


