CHAPTER 19. Assisted Reproductive Technology
OBJECTIVES
While studying the information covered in this chapter, the reader should attempt to:
■ Acquire a working understanding of assisted reproductive technologies, including oocyte transfer, intracytoplasmic sperm injection, gamete intrafallopian transfer, in vitro fertilization, and nuclear transfer.
■ Acquire a working understanding of types of infertility in the mare that may benefit from assisted reproductive techniques.
STUDY QUESTIONS
1. Describe the principles used in performing the following techniques, and explain circumstances that may favor use of one technique over the other:
a. Oocyte transfer
b. Intracytoplasmic sperm injection
c. Gamete intrafallopian transfer
d. In vitro fertilization
e. Nuclear transfer (cloning)
2. Describe procedures for harvesting ovaries from a mare after an untimely death and for transporting those ovaries to a laboratory for oocyte retrieval for assisted reproductive technology.
The term assisted reproductive technology (ART) covers a broad range of procedures but is most commonly used to refer to those procedures that use isolated oocytes to produce offspring. The ARTs most important in equine reproduction include oocyte transfer (OT), intracytoplasmic sperm injection (ICSI), gamete intrafallopian transfer (GIFT), in vitro fertilization (IVF), and nuclear transfer (NT; cloning).
With the exception of NT, these ARTs offer methods to obtain foals from mares that cannot provide embryos for transfer, or from reserves of stallion semen that are not adequate to achieve good pregnancy rates after standard intrauterine insemination. The main drawback to these techniques is that they require collection of oocytes from the valued donor mare, which is a semiinvasive procedure that requires specialized skill and equipment to perform. The technique of NT, or cloning, requires collection of only a skin biopsy sample from the donor animal and produces a foal with the same genetics as the donor. Major advances have been made in almost all fields of ART in horses over the last 10 years, making many of them commercially applicable.
OOCYTE RECOVERY
Recovery of Oocytes from the Dominant Preovulatory Follicle
In the live mare, oocytes may be efficiently recovered from the dominant preovulatory follicle after gonadotropin stimulation, via either transvaginal ultrasound–guided follicle aspiration (TVA), or puncture of the follicle via a needle placed through the flank. Aspiration of the stimulated preovulatory follicle provides a chance at only one or perhaps two oocytes per cycle but gives an oocyte with optimal developmental competence. Recovery rates from stimulated preovulatory follicles are high (70% to 80%) because the oocyte-cumulus complex has loosened from the wall of the follicle in preparation for ovulation (Carnevale and Ginther, 1995; Hinrichs et al., 1998).
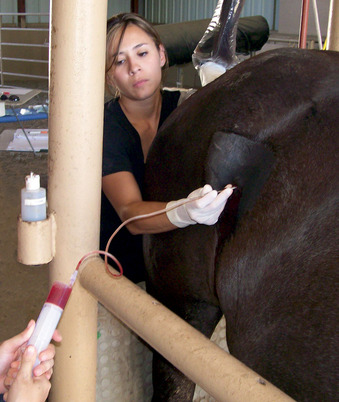
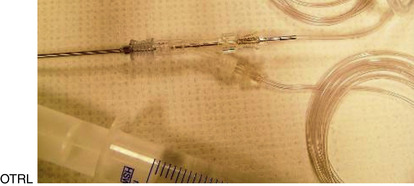
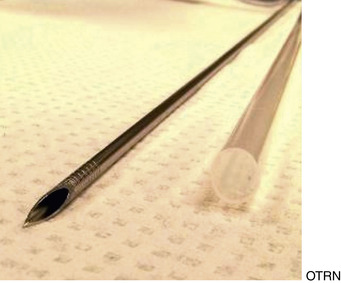
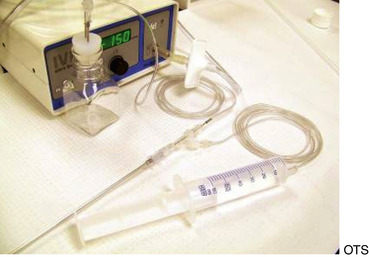
The major drawbacks to aspiration of the stimulated dominant preovulatory follicle are the necessity for monitoring of follicular growth, and accurate timing of gonadotropin administration in the mare, and the fact that only one or sometimes two follicles are available for aspiration. Superovulation regimens appear to be only minimally useful to increase the number of preovulatory follicles available for aspiration, as the ovary becomes flaccid after the first follicle is aspirated, and aspiration of subsequent follicles may be difficult (Maclellan et al., 2002). Limited superstimulation (of one or two additional follicles) may be useful but also may be difficult to achieve. Oocyte recovery from the dominant follicle may be performed simply with use of a 13- to 15-gauge, 20-cm needle guided through a cannula placed through the flank (Hinrichs et al., 1998). The ovary is manipulated with one hand per rectum, while the other hand manipulates the needle to puncture the follicle. The contents of the follicle are aspirated using a 50-mL all-plastic syringe and extension tubing (Figures 19-1 and 19-2).
Because the follicle has received gonadotropin stimulation, the oocytes recovered from aspiration of the stimulated, dominant preovulatory follicle have already resumed nuclear maturation and thus need only to be supported in culture to the time of predicted ovulation to mature completely.
Recovery of Oocytes from Immature Follicles
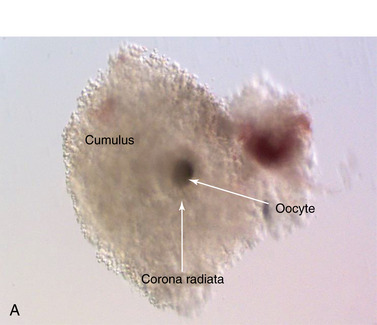
Oocytes may be recovered from nonmature follicles present on the ovaries of live mares; because of their small size, ultrasound–guided aspiration (TVA) is necessary to visualize the follicles. Oocytes recovered from these follicles are immature and must be cultured in the presence of gonadotropins to induce maturation in vitro (Figure 19-3). When all follicles on the ovary are aspirated, some oocytes are recovered from juvenile follicles, some from growing follicles, and some from follicles undergoing atresia. Thus, only 50% to 60% of recovered oocytes may be expected to mature on in vitro culture. Those that do mature in vitro have lower development rates after fertilization than those for oocytes obtained from stimulated preovulatory follicles. In addition, the recovery rate on aspiration of immature follicles has historically been low (typically 15% to 30%). For these reasons, most clinical ART programs have used aspiration of the stimulated preovulatory follicle to obtain oocytes for commercial oocyte transfer or ICSI (Hinrichs et al., 2000; Carnevale et al., 2005, 2007).
Recently, however, a good recovery rate (>50%) has been reported in a clinical program in which TVA of immature follicles was performed for oocyte collection. Oocytes were matured in vitro, fertilized with ICSI (see subsequent discussion), cultured to the blastocyst stage, and transferred to recipient mares. In this program, the increase in oocyte number per aspiration session overcame the effect of oocyte quality and resulted in a higher pregnancy rate per cycle (∼48%) (Colleoni et al., 2007) than was reported with a program that used ICSI with oocytes recovered on aspiration of only the dominant follicle (approximately 20% per cycle) (Carnevale et al., 2007). After the report of Colleoni and coworkers, our laboratory at Texas A&M performed TVA of immature follicles on Quarter Horse–type research mares every 2 weeks throughout the breeding season of 2008, for an average of 9 follicles aspirated and 5 recovered oocytes per mare per session. In vitro blastocyst production after ICSI averaged 63% per aspiration session (Jacobson, et al., in press).
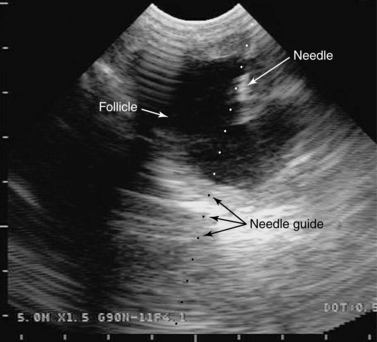
Transvaginal aspiration is performed with a transvaginal probe handle into which the ultrasound probe is mounted. The probe handle has within it a channel for the needle (see Figure 5-9). In the tranquilized mare, the probe is placed in the vagina, and the ovary is manipulated via palpation per rectum. A specialized needle, typically a 12- to 17-gauge double-lumen needle, is placed through the guide channel of the probe handle. The ovary is imaged through the vaginal wall (Figure 19-4) and manipulated by the hand per rectum so that the follicle is placed in the path of the needle as visualized on the ultrasound screen. The needle is then guided forward through the vaginal wall into the follicle (Figure 19-5), and the contents of the follicle are aspirated, typically with a vacuum pump (Figure 19-6). Obtaining a high recovery rate on aspiration of immature follicles necessitates manipulation of the follicle and needle during aspiration, to attempt to scrape the follicle walls, and repeated (up to 10 times) filling and emptying of the follicle with flush fluid. Recovery rates on aspiration of immature follicles with TVA appear to be technician-dependent; different laboratories consistently report very different recovery rates (from <15% to >50%) with this procedure.
Oocyte Recovery Post Mortem
If a mare dies or must be euthanized for medical reasons, then the ovaries may be removed and oocytes collected from them for ART. The ovaries are shipped to a laboratory equipped for oocyte collection and maturation. At the laboratory, the follicles are opened with a scalpel blade, and the interior tissue of the follicle (the granulosa cell layer) is scraped from the follicle wall with a bone curette. With use of a medium, the cells are washed into a Petri dish and the oocyte is located in the recovered cells. Oocyte recovery rates with this technique can be more than 80% with careful attention; typically, an average of 10 oocytes per mare are recovered from the ovaries of commercial mares processed post mortem. As for oocytes recovered from immature follicles ex vivo with TVA, these oocytes must be matured by culturing them in vitro in the presence of gonadotropins.
The factors that affect embryo development from oocytes recovered postmortem are still unclear; little information is available in this area. Factors that are likely to have an effect on the potential to produce a foal from these oocytes are the age of the mare, the length and severity of the illness, treatments administered, the method of euthanasia, the length of time the ovaries remain in the mare post mortem before removal, the temperature at which the ovaries are transported, and the length of time between death of the mare and processing of the ovaries. In our postmortem ICSI program, chronic illness of the donor mare and prolonged transport time (>12 hours) are associated with both lower blastocyst development in vitro (12% to 14% per oocyte subjected to ICSI versus 23% for mares not in these categories) and increased embryo loss rates of resulting pregnancies after embryo transfer. Carnevale et al. (2004), using oocyte transfer with oocytes recovered post mortem, reported a 36% embryo development rate if ovaries were processed within 1 hour of the mare’s death and a 10% development rate if the ovaries were processed 8 to 26 hours after death. With use of slaughterhouse tissue, we found a higher in vitro blastocyst development after ICSI in oocytes from ovaries processed within 6 hours postmortem than in those from ovaries processed 7 or more hours post mortem (Ribeiro et al., 2008). Cooling of ovaries to refrigerator temperature lowered maturation rates of recovered oocytes (Love et al., 2003).
On the basis of data from both clinical and research programs, current recommendations are to remove the ovaries from the mare with anesthesia (e.g., ketamine and xylazine) if possible; if not, then removal of the ovaries as soon after death as possible is recommended. We suspect that use of KCl for euthanasia may lower development rates but do not have controlled data on this. Ovaries should be transported to the laboratory at room temperature, preferably in less than 6 hours.
Clients should be informed that the rate of viable pregnancy from oocytes collected from mares post mortem is low. In the oocyte transfer program at Colorado State University, with oocytes collected post mortem, foals or pregnancies ongoing more than 70 days were obtained for six of 25 mares (24%; Carnevale et al., 2004). In our postmortem ICSI program at Texas A&M, from 2006-2009 we have obtained a total of 21 blastocysts from 16 mares, of which 13 produced pregnancies after transcervical transfer and 10 (63% per mare) have produced foals or currently ongoing pregnancies.
OOCYTE TRANSFER
OT is currently the most effective method to obtain a foal from a mare’s single isolated oocyte. It is conducted on a commercial basis in two centers (Colorado State University and Texas A&M University) and in a few clinical practices in the United States.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree