CHAPTER 17. Embryo Transfer
OBJECTIVES
While studying the information covered in this chapter, the reader should attempt to:
■ Acquire a working knowledge of reasons for performing embryo transfer in horses and the breed associations that permit use of this procedure.
■ Acquire a working knowledge of the methods for synchronizing ovulation in donor and recipient mares to be used in an embryo-transfer program.
■ Acquire a working understanding of techniques used to perform embryo transfer in horses.
STUDY QUESTIONS
1. Discuss the reasons behind selection of mares as embryo donors.
2. Discuss considerations used in selecting mares as embryo recipients.
3. Discuss the methods used to synchronize ovulation in a group of donor and recipient mares in an embryo-transfer program.
4. Discuss the techniques used to recover embryos from a donor mare.
5. Discuss the methods used for transfer of embryos to a recipient mare.
6. Discuss the factors that impact the success of an embryo-transfer program.
Aside from semen collection and artificial insemination, embryo transfer has become the most widely recognized assisted reproductive technique used in the horse. This brings to the equine practitioner an exceptional opportunity for practice expansion but also entails tremendous responsibility. What started about 25 years ago as a novel method of producing a foal from aged nonproductive broodmares has now expanded to become an elective procedure that is applicable to virtually all mares of reproductive age. This transition to a widely used elective procedure carries the responsibility commonly known as “above all else, do no harm” to the involved veterinarian.
Embryo transfer now lends itself to (1) obtaining foals from performance and show mares while they are competing; (2) multiple foals from the same mare in 1 year; (3) mares with nonreproductive health or musculoskeletal problems; and (4) mares with reproductive problems. In addition, through embryo transfer procedures, a mare that foals late in the year can be allowed early entry into the following breeding season without the loss of a foal. The use of embryo transfer has also enabled the development of more sophisticated advanced reproductive techniques such as intracytoplasmic sperm injection (ICSI) and nuclear transfer (cloning). Opportunities seem to be restricted only by our imagination.
Expectations for success have risen dramatically since the adoption of commercial embryo transfer. Results that were unimaginable just a few years ago are now achievable. For the sake of discussion, embryo recovery rates equal to or above the per-cycle pregnancy rate of a given stallion are expected. This translates to recovery of 100% of embryos in the uterus at the time of embryo retrieval. With regard to embryo-transfer success, the author (Hartman) contends that practitioners should set a goal of a 90% pregnancy rate at 12 days and 85% at 30 days after ovulation. One of the deceptive aspects of embryo transfer is that some degree of success may be achieved with relatively poor technique. The end result of this is an ever-present temptation to “cut corners” (i.e., to perform a uterine flush or embryo transfer in less-than-optimal conditions or using less-than-optimal supplies or media).
Conventional methods of embryo transfer have been described elsewhere by Colorado workers. This chapter discusses what has worked well in our practice and also some of the more controversial topics related to embryo transfer.
RECIPIENT MARE MANAGEMENT BEFORE TRANSFER
Management of a recipient herd is a daunting and labor-intensive task. This task begins in the fall of the previous year. During this period, it is important to acquire the total number of mares that are needed for the coming breeding season. New mares that may introduce contagious disease into the herd should not be added during the breeding season; the risk is too great among both the nonpregnant and pregnant mares. All individual mares should have a means of permanent identification to avoid any confusion regarding which mare is pregnant with which foal.
One of the most common complaints among mare owners concerns the quality of the recipient mares; therefore, selection of recipients that are reproductively sound is important, as is musculoskeletal soundness, dental health, vision, and behavior. The examination should include evaluation of udder health, because the ability to provide sufficient milk is a requisite for raising a healthy well-grown foal. All mares should pass a standard with regard to temperament and disposition. At the least, mares should be halter broke and gentle enough to catch in a small pen or stall. Fall and winter are also good times to manage vaccination, deworming, and Coggins tests, before mares are admitted into the recipient herd. Testing and vaccination for equine arteritis virus should be addressed where geographically indicated.
It is always a challenge to have sufficient mares with normal reproductive cyclicity in February (in the Northern Hemisphere) when the commercial breeding season begins. A good artificial lighting program is the most reliable method for ensuring early seasonal entry into reproductive cyclicity. For this protocol, mares are exposed to a total of 16 hours of light each 24-hour period beginning in the middle of November (see Chapter 3). The challenge centers on a good method of artificial lighting that can be used with large numbers of mares. The most common system involves holding the mares in large pens during the artificial lighting period. This holding can be stressful to the mares because they may be crowded and unable to access feed or water and are often exposed to inclement weather during confinement. Some studies discount the effect of stress on mares (Baucus et al., 1990); however, such studies generally examine a single stress insult rather than assessing cumulative stressors over time. Considerable anecdotal evidence points to the detrimental effect high stress has on such factors as maintenance of a corpus luteum (CL) and the mare’s ability to maintain early pregnancy.
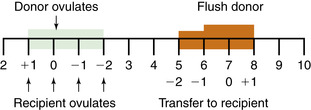
With the onset of breeding season, the goals for management of open recipient mares are twofold. First, knowledge of the precise day that each mare ovulates is necessary. This is best established with transrectal palpation and ultrasound of the reproductive tract during behavioral estrus. Examination should be done daily as the mare approaches ovulation and, oftentimes, for at least 1 day after ovulation. For record-keeping purposes, the day of ovulation is always considered day 0. The next day after ovulation, the mare becomes “+1”, the second day “+2”, and so forth. The commonly accepted window of synchrony between donor and recipient mares is such that recipient mares that are +4 to +8 days after ovulation are considered to be eligible to receive embryos (McCue et al., 2001; Vanderwall, 2000). An alternative method of describing synchrony is in regard to the recipient’s day of ovulation relative to that of the donor (Figure 17-1). The important concept here is that during this time the mare can respond to early signals involving maternal recognition of pregnancy and should also have adequate levels of circulating progesterone. Workers in the United Kingdom (Wilsher et al., 2005) successfully lengthened this window of synchrony, thereby enabling the use of recipients that were +9 to +10 days from ovulation. This was accomplished with the administration of meclofenamic acid (1 g per os, once daily) starting on day 9 after ovulation and continuing until day 7 after embryo transfer. With this protocol, the investigators were able to suppress the normal cyclical luteolytic response.
 |
| Figure 17-1 |
If determination of the day of ovulation becomes the primary focus, then a difficult aspect of recipient mare management, which is endometritis, is easily overlooked. Therefore, a second, equally important goal of management of open recipient mares is to ensure that they are reproductively sound and ready to receive an embryo. Begin by considering only mares that are 3 to 12 years of age for admission into the recipient herd. This should eliminate most mares with substantial chronic endometritis that would interfere with the ability to maintain a pregnancy to term. Next, evaluate the anatomy of the perineum and vulva. Very thin mares often have poor perineal anatomy, which is improved when they gain weight. If indicated, a Caslick’s operation should be performed. The importance of this cannot be overstated because bacterial contamination at the time of embryo transfer is a frequent cause of embryonic loss. It is apparent, then, that transrectal palpation and ultrasound examinations during estrus serve not only to establish the day of ovulation but also to evaluate the condition of the uterus. If indicated, uterine swabs should be procured for microbial culture. In our view, cultures should be taken only when the mare is in advanced estrus. The mare should have at least a 35-mm follicle that is beginning to mature, and the uterus should demonstrate substantial edema at the time of uterine culture. If these conditions are not met, false-negative culture results may be obtained. In addition, care should be taken not to contaminate the uterus during the process of obtaining the culture. Concurrently, control of delayed or inadequate uterine clearance is important. Florida workers recommend repeated oxytocin (20 IU given intravenously) injections to eliminate intrauterine fluid, but not more often than every 4 hours. These mares should be monitored for at least 1 day after ovulation to be certain no fluid is present in the uterus as they enter diestrus.
DONOR MARE MANAGEMENT BEFORE THE UTERINE FLUSH
Proper management of donor mares centers on the ultimate goal of recovering as clean an embryo as possible that is of a size that transfers well. With this in mind, practitioners should realize that contrary to conventional thought, donor mares with endometritis become pregnant on a regular basis and an embryo is commonly found in a very dirty flush. Regardless of the number of times the embryo is washed, getting these embryos to thrive after embryo transfer is difficult. Therefore, a prudent practice is to make sure the donor mare’s uterus is free of microorganisms at the time of insemination. Once-daily transrectal palpation and ultrasound examinations allow the practioner to follow follicular development and ovulation and, just as important, to monitor the conditions of the uterus. The time and effort spent keeping the donor mare’s uterus clean are rewarded with increased rates of successful embryo transfer. Keep in mind that bacterial contamination of the donor’s uterus can come from many sources. Among these are inadvertent contaminations during the breeding process, contaminated semen, and, increasingly commonplace, residual infection and endometritis from previous reproductive procedures.
Multiple embryo transfers on the same mare during a breeding season, oftentimes at different farms and by different veterinarians, are becoming more common. Colorado workers (Carnevale et al., 2005) analyzed the effects of repeated inseminations and embryo transfer attempts on uterine health and found an association with increased chronic inflammatory changes. These workers concluded that mares were susceptible to the additive effects of repeated uterine insults. In clinical practice, substantial evidence shows that repeated breeding and embryo transfer attempts yield an increased likelihood of acute bacterial endometritis, in addition to chronic inflammation. Therefore, proper donor mare management necessitates continued vigilance to guard against endometritis. Donor mares may need uterine cultures on a regular basis throughout the breeding season. However, the overuse of intrauterine antibiotic therapy should be discouraged because this may lead to overgrowth of yeasts or fungi. One of the most effective ways to keep a mare’s uterus in a hygienic state is through effective uterine lavage along with ecbolics to aid in uterine clearance.
One factor that affects embryo transfer success is the size of the embryo at the time of transfer to the recipient mare. Large embryos present their own set of problems. For example, they are somewhat more fragile, and a transfer apparatus that can accommodate the larger embryos is sometimes difficult to find. On the other hand, if a donor mare’s uterus is flushed too early, the embryo may still be in the oviduct and not available to traditional recovery attempts. In fact, it is not uncommon for a mare to end up pregnant after being flushed for embryo retrieval. Therefore, selecting the correct flush day is of paramount importance. The ideal embryo size is usually achieved by 7 days after ovulation, at which time its diameter is approximately 400 to 500 μm. However, the optimal approach is not so simple as to automatically flush all mare uteri at 7 days after ovulation. The timing of embryo entry into the uterus has been established to be somewhat variable (Battut et al., 2000), ranging between 144 hours (6 days) and 156 hours (6.5 days) after ovulation is first detected. Examination of the CL in the periovulatory period is important in that scheduling a 7-day flush date from a recent ovulation often results in a smaller-than-expected embryo. Several factors seem to influence this variability in embryo size: (1) the age of the donor mare; (2) the inherent fertility of the stallion; (3) the timing of breeding relative to the time of ovulation; and (4) variation in time between ovulation and fertilization. In our practice, most reproductively normal mares are flushed 7 days after ovulation is detected. A simple way to plan is to consider the day of the week when planning for the embryo flush attempt. For example, if Monday is the day ovulation is first detected (day 0), the mare’s uterus is flushed on the following Monday for a 7-day flush. Among the exceptions to this are old mares (18 years or older), mares bred to subfertile stallions or older stallions, and mares bred with frozen semen, which are all flushed on day 8.
During the week between ovulation and the uterine flush, donor mares should be kept as stress-free as possible. Evidence shows that performance mares in training may have reduced embryo recovery rates (Mortenson et al., 2009). These reduced rates may result, in part, from the fact that some mares have a significant increase in body temperature during strenuous physical activity that can have an adverse affect on embryonic development or survival.
Older mares and mares that ovulate small follicles may benefit from progesterone supplementation during the first week after ovulation. The most practical way to do this is with administration of altrenogest (0.044 mg/kg orally, once daily) until the embryo recovery attempt is performed. Other candidates for progesterone supplementation are the mare that has multiple follicles with asynchronous ovulations (separated by 3 to 4 days) and the mare that does not develop a prominent CL after ovulation.
UTERINE FLUSH FOR EMBRYO RECOVERY
Methods used for embryo recovery have been described previously (McCue et al., 2001; Vanderwall, 2000). In general, recovery is accomplished with three to four transcervical uterine lavages, with the effluent fluid passed through a filter that captures the retrieved embryo.
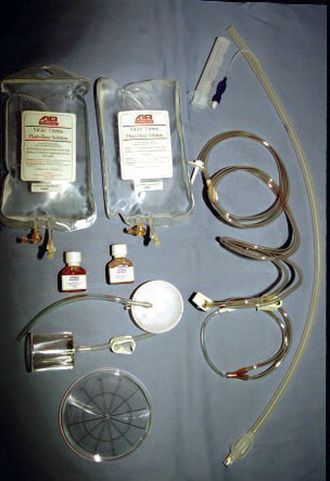
A wide variety of embryo transfer supplies are readily available from commercial distributors for equine embryo-transfer procedures (Figure 17-2; Table 17-1). Product selection is largely based on personal choice. Most of these supplies have been sterilized with γ-irradiation before sale. After use, the decision of whether to reuse these supplies for subsequent embryo-transfer procedures is again up to the individual. Resterilization of supplies can be problematic. Only the most expensive catheters may be steam autoclaved. Gas sterilization presents a problem with regard to the amount of time after the sterilization that is safe before exposure of an embryo to sterilized supplies. Any disinfectants that are used in a washing program may also be potentially harmful to an embryo. For these reasons, all supplies in our practice are used only once for embryo transfer procedures and then reused thereafter for therapeutic uterine lavage.
| Full Line of Embryo Transfer Products and Supplies | |
| Agtech Inc, USA 8801 Anderson Avenue Manhattan, KS 66503 Toll-Free: 800-367-4016 Direct: 785-776-3863 Fax: 785-776-4295 www.agtechinc.com | Bioniche Animal Health 1551 Jennings Mill Road, Suite 3200A Bogart, GA 30622 Toll-Free: 800-265-5464 Fax: 613-966-4177 www.bionicheanimalhealth.com |
| Exodus Breeders Corporation 5470 Mount Pisgah Road York, PA 17406 Toll-Free: 877-396-3874 Fax: 717-252-4221 www.exodusbreeders.com | IMV USA 11725 95th Avenue North Maple Grove, MN 55369 Toll-Free: 800-342-5468) Fax: 763-488-1888 www.imv-technologies.com |
| Har-Vet PO Box 39 Spring Valley, WI 54767 Toll-Free: 800-872-7741 Fax: 800-227-1324 www.har-vet.com | Pets Professional Embryo Transfer Supply Inc PO Box 188 Canton, TX 75103-0188 Phone: 903-567-4536 www.pets-inc.com |
| Partner Animal Health 3560 Pine Grove Avenue #227 Port Huron, MI 48060 | Veterinary Concepts PO Box 39 Spring Valley, WI 54767 |
| General Laboratory and Chemical Supplies | |
| Fisher Scientific 711 Forbes Avenue Pittsburgh, PA 15219 Toll-Free: 800-766-7000 www.fisherscientific.com | Sigma Chemical Co PO Box 14508 St Louis, MO 63178-9916 Toll-Free: 800-325-3010 Fax: 800-325-5052 www.sigma-aldrich.com |
| VWR Scientific PO Box 7900 San Francisco, CA 94120 Toll-Free: 800-932-5000 www.vwrsp.com | |
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree