CHAPTER 14. Semen Preservation
OBJECTIVES
While studying the information covered in this chapter, the reader should attempt to:
■ Acquire a working understanding of the advantages and disadvantages of breeding with transported, cooled, or frozen equine semen.
■ Acquire a working understanding of techniques and procedures used to process equine semen for cooling or freezing and to manage or breed mares with cooled or frozen equine semen.
STUDY QUESTIONS
1. List the ingredients in semen extenders that protect equine sperm against cold shock.
2. Discuss desirable cooling rates for minimizing damage to sperm in extended equine semen.
3. List the steps (in order) to be followed to prepare equine semen for cooling and shipment.
4. List the steps (in order) to be followed to freeze equine semen.
5. Discuss the reasons for, methods of, and expected fertility related to cryopreservation of epididymal sperm.
6. Discuss the methods for inseminating mares with cooled or cryopreserved semen.
EQUINE SEMEN PRESERVATION: COOLED AND FROZEN SEMEN
Most equine breed registries within the United States condone pregnancies achieved via artificial insemination. Many of the registries also permit artificial insemination with cooled or frozen semen. Before embarking on an artificial insemination program, particularly with cooled or frozen semen, one must obtain the current regulations for the breed registry of interest, because regulatory allowances may change without widespread notification.
Transported, cooled semen is used increasingly for breeding purposes in the United States, and most U.S. breed registries currently permit the use of cooled or frozen semen for off-site use. Preserved semen may be more commonly used outside the United States. It has been used on a large scale for artificial insemination in China, where hundreds of thousands of mares have been inseminated with transported frozen or cooled semen in recent years. In European countries, preserved semen is used predominantly for Warmblood, draft, and Standardbred breeds.
Sperm are sensitive to many environmental factors, including temperature, light, physical damage, and a variety of chemicals. Accordingly, the semen preservation process begins with the semen collection process; one should use precautions to thwart any environmentally induced injury to sperm (see Chapter 12). Damage to sperm from cold shock is attributed primarily to temperature-induced membrane perturbations and oxidative stress.
Cooling of extended semen for transport is generally successful if the initial quality of the semen is good, a proper storage technique is used, and insemination is not delayed beyond 24 hours. For some stallions, good pregnancy rates can be obtained with semen stored for 48 to 72 hours before insemination.
Currently, frozen preservation of stallion sperm generally yields poorer fertility than cooled storage and is also far less successful than frozen preservation of sperm from dairy bulls. Direct extrapolation of techniques used to freeze bull semen has yielded discouraging results with stallion semen, an outcome probably related to compositional differences between the sperm of the two species. Research has revealed that sperm of dairy bulls are unusually resistant to the effects of cryopreservation. This phenomenon is probably attributed to years of careful selection of bulls with sperm capable of surviving the freezing and thawing processes, a practice not followed in horse-breeding circles.
The economic incentives for improving techniques of semen preservation in stallions are becoming increasingly evident, as major breed registries now permit its use. Previously, limited financial support for research in this field had slowed progress in development of superior methods for preserving stallion semen, in both cooled and frozen forms. Advancements now being made in this area may lead to more prolonged storage intervals for cooled semen and successful cryopreservation of semen from a larger percentage of stallions.
GENERAL CONSIDERATIONS
Preservation of semen begins with the actual collection process. To ensure that sperm quality is optimized, semen should be collected as described in Chapter 12. Regardless of the technique of semen preservation to be used, semen must be placed in a suitable extender within a few minutes after collection from the stallion. To maximize success with preserved semen, one should screen for ejaculates of poor quality. If the quality of fresh stallion semen is poor (see Chapter 13) or if fertility achieved by breeding with fresh semen is poor, successful results are highly unlikely to be obtained by breeding with preserved semen.
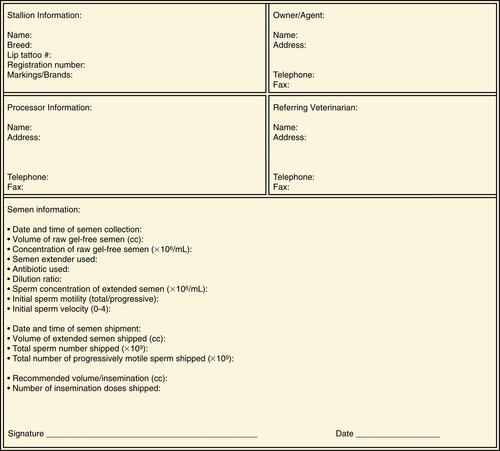
Figure 14-1 provides an example of a processing form to be sent with the transported, cooled semen as a method of quality control. Copies of this form can be kept in a log book for the stallion owner to help maintain accurate records of mares being bred.
COOLED SEMEN
Extended semen from fertile stallions can oftentimes be stored in a cooled state for hours to days before insemination, without a significant reduction in pregnancy rate. Guidelines for maximizing the longevity of sperm viability after cooled storage follow.
Dilute Semen with a High-Quality Extender
Semen extenders contain protective ingredients that permit survival of sperm outside the reproductive tract. Lipoproteins, such as those contained in milk or egg yolk, protect sperm against cold shock by stabilizing cellular membranes. Metabolizable substrates, such as glucose, provide a plentiful source of energy for sperm. Antibiotics are added to extenders to retard or eliminate growth of bacterial organisms. The osmotic pressure and pH of extenders can be adjusted to maximize sperm survival. The osmolarity of milk-based extenders should be between 300 and 400 mOsm/L, with 350 mOsm/L considered to be optimal. The pH of semen extenders can probably range from approximately 6.5 to 7.2 without affecting the longevity of sperm viability during storage, but a pH of 6.7 to 6.9 may optimize sperm track linearity (i.e., a computerized motility assessment measure of the straightness of sperm movement). Extenders may be homemade formulations (see Table 12-1) or commercially available preparations (see Table 12-2). Nonfat dried milk solids–glucose (NFDMS-G) extender maintains better sperm motility in cooled semen than does heated skim milk extender, although fertilizing capacity may not differ between the two extenders. The NFDMS-G extender also maintains cooled sperm motility better than a sucrose-bovine serum albumin extender. No conclusive studies have been done to determine whether cream-gel– or egg yolk–based extenders are more or less desirable than milk-based extenders for enhancing either sperm viability or fertility in cooled equine semen. Milk-sugar extenders are the most popular formulations in the United States, and milk-based extenders are more commonly used worldwide than are egg yolk–based extenders. A commercial extender that has become quite popular in recent years in the United States is the French-developed INRA 96 extender (available though IMV Technologies, Maple Grove, Minn.). This extender contains native phosphocaeseinate derived from milk as the sole source of protein-based sperm membrane protection against cold shock. Other milk components were excluded from the composition of this extender because some were considered to be detrimental to sperm function.
Ideally, semen should be mixed with a prewarmed (37°C) extender within 2 to 5 minutes after ejaculation. A minimum ratio of 1:1 (extender to semen) is recommended for immediate inseminations. If semen is to be stored for a period of 2 to 4 hours or longer before insemination, greater dilution (i.e., a higher extender-to-semen ratio) is usually necessary. A final concentration of 25 to 50 million sperm/mL in extended semen generally maximizes sperm survivability in vitro. To maximize sperm survival, a minimum of a 1:4 (i.e., 1 part semen to 4 parts extender) dilution should be obtained to ensure that the final seminal plasma concentration in extended semen is less than 20%. For dilute ejaculates (<100 million sperm/mL), centrifugation of the semen may be necessary to remove excess seminal plasma; resuspension of the sperm in extender may be necessary to ensure that no more than 20% seminal plasma remains in the extended semen and the final sperm concentration is at least 25 million sperm/mL.
The seminal plasma of some stallions can have a detrimental effect on sperm viability, even when the seminal plasma concentration is adjusted to 5% to 10% (volume/volume [v/v]) in the extended semen. In these circumstances, centrifugation of the semen after its initial dilution with extender can become necessary, followed by removal of virtually all of the seminal plasma. The pellet of sperm can then be resuspended in extender preloaded with sperm-free seminal plasma (5% to 10% [v/v]) from a fertile stallion that is known to possess good quality seminal plasma. In this circumstance, it is important to ensure that the seminal plasma of the donor is free of organisms that cause transmissible disease, such as equine viral arteritis, equine infectious anemia, or contagious equine metritis. Although sperm are best preserved when customary milk-based extenders contain 5% to 10% seminal plasma, a centrifuged sperm pellet devoid of seminal plasma can be placed in an extender type developed at Cornell University (Kenney extender with modified Tyrode’s medium [KMT]). The composition of this extender (Table 14-1) is such that it eliminates the need for seminal plasma in the extender. Fertility trials with semen centrifuged to remove seminal plasma, followed by dilution of sperm in KMT extender and cooled storage, have yielded excellent results.
| MTM, Modified Tyrode’s medium. | |
| *Modified from Padilla AW, Foote RH: Extender and centrifugation effects on the motility patterns of slow-cooled stallion spermatozoa. J Anim Sci 1991;69:3308-3313. | |
| NaCl | 420 mg |
| KCl | 187 mg |
| NaHCO 3 | 210 mg |
| NaH 2PO 4 | 5 mg |
| Na lactate (60% syrup) | 0.31 mL |
| CaCl 2 • 2 H 2O | 29 mg |
| MgCl 2 • 6 H 2O | 8 mg |
| HEPES | 238 mg |
| Na Pyruvate | 11 mg |
| Amikacin sulfate | 100 mg |
| Potassium penicillin G | 1 × 10 5 units |
| Bovine serum albumin (fraction V) | 600 mg |
| Deionized distilled water | qs 100 mL |
| Kenney extender containing modified Tyrode’s medium | Mix MTM with a Kenney-type extender at a ratio of 35% MTM and 65% Kenney-type extender |
COOL SEMEN TO REFRIGERATED TEMPERATURE FOR STORAGE
Extended semen should be promptly removed from the incubator (37°C) because extensive sperm death occurs within a few hours if sperm are maintained at this temperature. Cooling extended semen to 4°C to 8°C for storage is superior to storage at room temperature (i.e., 20°C to 25°C) for breeding 1 to 2 days later. The longevity of sperm viability is probably improved by storage at near-refrigeration temperature compared with storage at higher temperatures because of a corresponding reduction in metabolic activity. Sperm fertilizing capacity is often maintained for 24 to 48 hours or longer when extended semen is stored at refrigerated temperature. Normal fertility has been reported after refrigerated storage of semen for periods of 72 to 96 hours for selected highly fertile stallions. If semen is stored at room temperature, fertility is often reduced after 12 to 24 hours. Some studies have suggested that a storage temperature of approximately 15°C may be superior for semen from some stallions, but more research is needed to determine whether this is the case.
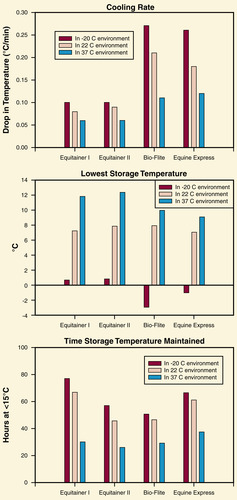
When stallion semen is extended for transport and subsequent artificial insemination, a rapid change to temperatures of less than 18°C to 20°C causes sperm to undergo cold-shock damage. Currently, only passive cooling/transport systems are commercially available for use with stallion semen. Passive cooling systems generate variable rates of cooling (i.e., cooling rates become progressively slower as the target semen temperature is approached). Cooling rates may also vary according to environmental temperatures, initial temperature of semen, and volume of extended semen being cooled (Figure 14-2). Experimental studies have shown that an initial cooling rate of –0.3°C/min is most desirable for maximizing sperm viability. This cooling rate is achieved with the Equitainer I or Equitainer II (Hamilton Research Inc, South Hamilton, MA). Cooling rates from 20°C to 8°C are thought to be more critical for stallion sperm survival than are cooling rates from 37°C to 20°C, and a range of –0.05°C/min to –0.17°C/min is currently recommended to maximize the maintenance of sperm motility. Final storage temperatures of 4°C to 8°C are currently thought to be superior to temperatures of 0°C to 2°C, but more studies are needed to determined whether this the case.
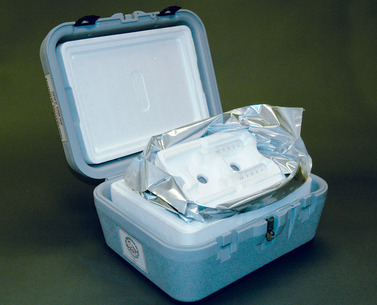
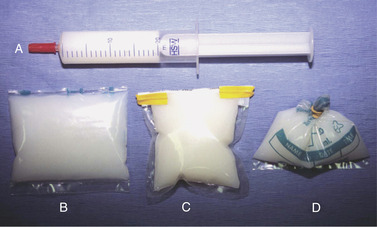
Commercial systems for semen storage, such as the Equitainer I, Equitainer II, and Equitainer Clipper (Hamilton Research Inc) (Figure 14-3, Figure 14-4, Figure 14-5, Figure 14-6, Figure 14-7, Figure 14-8 and Figure 14-9); the Equine Express II (Exodus Breeders Corporation, York, PA) (Figure 14-10); and the Equine Semen Transporter (EST-SH, EST XL–SH, EST-S, and EST XL; Plastilite Corporation, Omaha, NE; Figure 14-11), greatly simplify transport of cool-stored stallion semen. Semen is generally packaged in either plastic bags or all-plastic syringes before loading into semen shipment containers (Figure 14-12). The package type is dependent on the style of container used for semen transport. Heat sealing of packages provides a nice product, but should be done in such a manner that the heat-sealing process does not damage semen (Figure 14-13 and Figure 14-14). Labels should be affixed to packaged semen so that pertinent information regarding the stallion identification, semen collection date, and mare to be inseminated is easily identified (Figure 14-15). Additional information should be made available on the accompanying semen transport form (see Figure 14-1).
 |
| Figure 14-7 All components are loaded in the order shown in Figure 14-6 into a plastic bag to be inserted into the Equitainer, permitting easy removal of contents when the mare is to be bred. |
Regulations for interstate shipment of semen are not well defined, so packaged semen is usually transported via air carrier throughout the United States without accompanying health certificates. Authorized shipment of semen outside the United States requires that specific regulations of the importing country be met. Health authorities responsible for importation of semen into other countries should be contacted to ensure that requirements are met before arrangement for semen transportation is made. Some countries require that extensive health screening (e.g., serologic testing, culturing of reproductive tract or semen, vaccination, inspection of premises and animals by regulatory officials) be performed before semen is collected for preservation.
Quarantine measures sometimes must be used for the stallion, farm, and mares maintained at the farm where the stallion stands to meet semen importation requirements. Cooled semen has been successfully imported into the United States from abroad for prompt insemination of mares, but considerable regulatory requirements must be met before shipments are authorized.
Cool-transported semen that is transported “counter-to-counter” by commercial airlines may be subjected to radiographic security screening. One study revealed no adverse effects of x-radiation at doses up to 10 μSv (an exposure level similar to that of conventional airport security screening systems) on equine sperm motility (either initial motility or longevity of motility), sperm morphology, or fertility of irradiated semen. Mares bred with x-irradiated semen also delivered normal foals at term. However, recommendations for screening luggage on commercial flights in the United States may result in new radiographic screening requirements that could increase the amount of radiation exposure to 300 times or more than that currently used. Effects of exposure of horse semen to this amount of radiation remain unstudied. Hamilton Thorne Research Inc (Beverly, Mass.), provides a lead shield for their reusable transport systems that may protect semen from potential radiation damage; however, the shield overlies the top of the container and does not prevent radiation exposure to the sample if the transport container is exposed to radiation from the side. The container is labeled to caution airport luggage screening personnel to only allow the container to be opened for less than 2 minutes during examination. The manufacturer has found that opening the container for less than 2 minutes does not interfere with the cooling rate or holding temperature of the stored extended semen. Plastilite Corp provides lead-foil shields for their reusable and disposable semen shipping systems.
The most accurate method for determining the inseminate dose (i.e., sperm number) necessary for transport for breeding is to conduct semen cooling trials for individual stallions. The semen is diluted in an appropriate extender as described previously and cooled for 24 hours in semen transport containers to be used by farm management personnel. The cooled semen sample is gently remixed after this cooling period, and an aliquot is warmed to 37°C. Sperm motility is evaluated 15 minutes after warming, and the percentage of progressively motile sperm obtained is used as a guideline to ensure that future shipments provide a minimum of 500 million progressively motile sperm after 24 hours of cooling. For example, if after 24 hours of cooling, the progressive sperm motility is 50%, 1 billion total sperm would need to be prepared for shipment to ensure that an insemination dose of 500 million progressively motile sperm was available for breeding a mare. The procedure can also be performed on semen cooled for 48 hours if demand for 48-hour cooled semen is suspected. If justified, more extensive studies of cooled semen can be conducted by reference laboratories, such as objective measurement of sperm motion, chromatin quality, membrane integrity, intact acrosomes, and morphology after cooled storage.
It has become common practice for some stallion owners/managers to prepare two insemination doses of extended semen for shipment: one to be used for an initial insemination on arrival, and one to be held for insemination again the next day. For many stallions, the longer the semen is held at a refrigerated temperature, the poorer sperm motility becomes. In addition, fertility of semen cooled for 48 hours tends to be reduced compared with that of semen cooled for only 24 hours. Except for special cases, we believe it is generally better to inseminate a mare with the transported semen as soon as practical after it arrives rather than to wait for breeding or to inseminate the mare twice, 24 hours apart, with the semen in that shipment. More research must be performed before convincing evidence shows that fertility is improved by holding a second insemination dose for breeding the day after the first insemination. Certainly, if two large doses of good-quality semen arrive, breeding the mare twice at 12- to 24-hour intervals might be advantageous. However, if sperm motility is poor when the semen arrives, it is unlikely that the second dose will survive an additional 12 to 24 hours of cooling. If the practitioner chooses to hold semen for breeding again the next day, precautions should be taken to maintain the cooled semen at 4°C to 6°C until the time of the second insemination. To ensure that the temperature of the second dose of semen left in the shipping container does not increase above 10°C, causing premature sperm death, the remaining semen dose can be repackaged and the container placed in a refrigerator (4°C to 6°C) until it is opened for breeding the next day. Alternatively, the remaining packaged semen can be removed from the shipping container, wrapped in an insulting paper towel, and placed in a refrigerator that is set at approximately 4°C.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree