CHAPTER 12. Semen Collection and Artificial Insemination with Fresh Semen
OBJECTIVES
While studying the information covered in this chapter, the reader should attempt to:
■ Acquire a working knowledge of procedures used for collection of semen from stallions.
■ Acquire a working knowledge of proper breeding management of stallions and semen processing and handling techniques used in artificial insemination programs.
STUDY QUESTIONS
1. Discuss advantages and disadvantages of artificial insemination programs compared with natural breeding programs for horses.
2. Describe important components of a breeding shed to be used in an equine artificial insemination program.
3. Give advantages and disadvantages of the commonly used equine artificial vaginas.
4. Describe the technique for preparation of an artificial vagina for semen collection in the stallion.
5. List the advantages of use of a breeding phantom for semen collection.
6. Describe the role of semen extenders and temperature in maintenance of equine sperm viability in vitro.
7. Discuss the proper insemination dose (number of sperm and insemination volume), insemination timing, and insemination technique for artificial insemination in the mare.
8. Discuss indications for and advantages of low-dose insemination of horses.
9. Describe the technique for deep horn insemination with small inseminate volumes.
10. Describe the technique for hysteroscopic insemination directly onto the oviductal papilla.
Proper application of artificial insemination (AI) in an equine breeding program can dramatically improve operating efficiency and increase the availability of sires to the general public. This chapter addresses advantages and disadvantages of AI programs and provides a detailed description of the techniques involved.
Few breed registries in the United States do not currently permit the use of AI (e.g., the Jockey Club [Thoroughbreds]). The allowances and limitations regarding storage and transport of semen vary considerably among the breed registries that permit the use of AI. One should contact specific breed registries before instituting an AI program to determine restrictions that might limit registration of foals produced.
ADVANTAGES AND DISADVANTAGES
AI offers numerous advantages over natural mating. For instance, division of an ejaculate into several insemination doses permits more efficient use of stallion semen, provided that the stallion has normal fertility. Accordingly, the number of mares that a stallion can impregnate during a breeding season or calendar year may be increased several-fold. The availability of stallion semen to mare owners is likewise increased within those breed organizations whose bylaws permit preservation and transport of stallion semen. Addition of antibiotics to semen extenders for AI minimizes venereal transmission of bacterial diseases to the mare where the stallion serves as a carrier. Transmission of potential pathogens from mare to stallion can also be reduced. Semen extenders contain supportive and protective factors for sperm that may improve the pregnancy rates of certain stallions. Use of a breeding phantom for collection of semen reduces the risk of breeding injuries. Collection of semen with an artificial vagina also allows scrutiny of semen quality before insemination and allows early detection of problems that may adversely affect the fertility of stallions. The development of low-dose insemination techniques further enhances the number of mares that can be bred with an ejaculate and can improve pregnancy rates for some subfertile stallions.
Certain disadvantages are inherent to AI programs. The success of such programs requires heightened knowledge and skill on the part of the stallion manager because ejaculated sperm are very susceptible to environmental injury. Improper semen collection, handling, processing, and insemination technique can lower pregnancy rates. Expenses related to the purchase of necessary equipment and supplies for AI can increase overhead costs of the breeding program. However, expenses incurred on a per mare basis are usually decreased because of the multiple inseminations possible with a single ejaculate. Another disadvantage is the somewhat increased risk of human injury during the process of semen collection with an artificial vagina (AV). Therefore, proper training of persons involved in the semen collection process is essential. Further training is needed to reach a level of competence to achieve satisfactory pregnancy rates with low-dose insemination techniques because semen processing and handling requirements are heightened. Finally, equipment costs for hysteroscopic insemination are relatively expensive compared with those required for manual insemination practices.
SEMEN COLLECTION
The semen collection procedure is an essential part of the AI program. Facilities and equipment that permit safe and efficient collection and handling of the semen are discussed.
Artificial Vagina Selection
A properly constructed and prepared AV increases the efficiency of semen collection in AI programs and optimizes the quality of ejaculated semen. Several well-designed AVs are available. Each type has distinct attributes and peculiarities, so AV selection is based on specific requirements and personal preference. AVs have also been homemade by users to meet their specific needs. When contemplating the purchase of an equine AV, one should consider initial and maintenance costs, durability, weight, temperature maintenance, and sperm losses incurred during semen collection.
Missouri Model Artificial Vagina
The Missouri model AV (NASCO, Ft. Atkinson, WI) (Figure 12-1) probably is the most widely used AV in the United States. It is composed of a double-walled rubber liner that contains a permanently sealed water chamber and a leather carrying case. This AV is relatively inexpensive, lightweight, and easy to assemble and clean. It has fairly good heat retention, and because the glans penis should be beyond the water jacket at the time of ejaculation, internal temperature of the AV may exceed the 45°C to 48°C sperm tolerance threshold without causing heat-related injury to ejaculated sperm. This property can be advantageous for stallions that prefer higher temperatures. This AV is equipped with an air valve so that it can be pressurized with water alone or with air and water to reduce its weight. Because the liner is made of heavy rubber and the water chamber is permanently sealed, there is little chance of water leakage contaminating the semen sample during collection. Latex liners are available for this AV in two lengths (16 inches and 22 inches). The occasional stallion with a large penis may prefer the longer liner.
 |
| Figure 12-1 (From Varner DD, Schumacher J, Blanchard TL, et al: Diseases and management of breeding stallions, Goleta, CA, 1991, American Veterinary Publications, 118.) |
Japanese (Nishikawa) Model Artificial Vagina
The Japanese (Nishikawa) model AV (Figure 12-2) is composed of a small, rigid aluminum case and a single rubber liner, so it is lightweight and easy to maneuver. The Japanese model is no longer available commercially in the United States, but the Har-Vet model (Har-Vet, Spring Valley, WI) closely resembles the Japanese model, except that it has a plastic rather than an aluminum casing. The AV is easy to assemble and clean. Another attribute of this AV is the direct attachment of a semen receptacle to the casing. This design permits the ejaculate to be discharged directly into the receptacle, allowing only minimal contact of semen with the rubber liner. The liner must be secured tightly to the case with rubber bands before water is added to the chamber between the liner and the case. Water can leak out if this seal is not tight, reducing AV pressure and increasing the risk of water contamination in the ejaculate. The liner should be checked for defects before use because pinpoint holes in the rubber liner can develop, resulting in water leakage into the AV lumen.
 |
| Figure 12-2 (From Varner DD, Schumacher J, Blanchard TL, et al: Diseases and management of breeding stallions, Goleta, CA, 1991, American Veterinary Publications, 119.) |
Colorado Model Artificial Vagina
The original Colorado model AV is composed of two independent rubber liners and a heavy plastic case covered by a leather collar. It is more cumbersome to use than the previous two AVs but offers good heat retention. Because it has two rubber liners between the water chamber and the AV lumen, the likelihood of water contamination of the semen sample is greatly reduced. The water jacket of this AV is longer than the penis; therefore, the temperature must be carefully regulated to prevent undue heat damage to ejaculated sperm.
CSU Model Artificial Vagina
The CSU model AV (Animal Reproduction Systems, Chino, CA) (Figure 12-3) and Lane model AV (Lane Manufacturing, Denver) are modified versions of the original Colorado model. Both models are lighter than the original and have a rigid handle to facilitate AV manipulation. These versions have the advantages described previously for the Colorado model and come with an assortment of accessories, including several AV liners, semen filters and collection bottles, a thermometer to monitor internal temperature of the AV, and an insulated protector cone to cover the semen receptacle during the semen collection process.
 |
| Figure 12-3 (From Varner DD, Schumacher J, Blanchard TL, et al: Diseases and management of breeding stallions, Goleta, CA, 1991, American Veterinary Publications, 120.) |
Polish Model Artificial Vagina
The Polish model AV was designed as an open-ended AV for collection of only the sperm-rich fraction of the ejaculate from breeding stallions. This method of semen collection increases sperm concentration and, likewise, reduces the contribution (volume) of seminal plasma in ejaculates. The first three jets of an ejaculate contain 60% to 80% of the sperm present in total ejaculates, yet seminal volume is reduced more than 50%. The result of this semen-fractionation step is a significant increase in sperm concentration, sometimes exceeding 0.6 to 1 × 10 9 sperm/mL. The technique is considered useful for semen preservation programs because the sperm concentration can be increased without centrifugation and toxic influences of seminal plasma on sperm viability may be diminished. Collection of semen with this method often reduces contamination of ejaculates, whether by bacteria from the exterior of the penis, by urine from stallions affected by urospermia, or by blood from stallions with a penile injury. Removal of the coned end of the Missouri model AV (Figure 12-4) or shortening of the CSU model AV works well for this purpose. The glans penis is allowed to protrude from the AV, and as the semen is ejaculated, it is collected through a funnel system attached to semen receptacles or in urethral prosthetic devices attached to a bag (Figure 12-5), or by catching semen in individual receptacles.
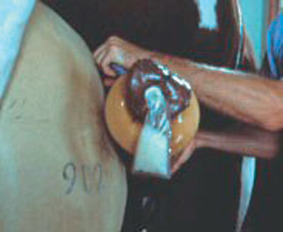 |
| Figure 12-5 (From Varner DD, Schumacher J, Blanchard TL, et al: Diseases and management of breeding stallions, Goleta, CA, 1991, American Veterinary Publications, 122.) |
Two other AVs are commercially available. The French (INRA) model (IMV International Corp, L’Aigle, France) is similar to but smaller than the Colorado AV and has two handles. The Roanoke artificial vagina (Roanoke AI Labs, Roanoke, VA) has a short, variable-diameter rigid casing designed to facilitate manual stimulation of the upper shaft of the penis.
Artificial Vagina Maintenance
All AV components that come in contact with ejaculated semen must be nonspermicidal. Reusable items must be cleansed properly to render them chemically clean, then dried, and, if possible, sterilized between uses. Soaps or disinfectants should not be used to clean AV liners because the residue can be toxic to sperm during subsequent semen collections. The rubber AV liners should be cleaned with hot running water soon after use. If smegma within an AV is allowed to dry before cleaning, the chore becomes more difficult. Cleaned rubber liners can be submerged in ethyl or isopropyl alcohol for 0.5 to 24 hours for disinfection followed by air-drying in a dust-free cabinet. Any of the latex rubber liners can be gas sterilized with ethylene oxide as long as an adequate “air-out” period is allowed (i.e., 48 to 72 hours). Sterile, nontoxic disposable equipment should be used when possible to avoid chemical contamination of ejaculates or horizontal transmission of disease. Plastic disposable AV liners, with or without attached semen receptacles, are commercially available for most AVs. This equipment helps ensure a clean, nontoxic method of semen collection. Unfortunately, some stallions do not readily accept AVs fitted with a disposable plastic liner and seem to prefer the feel of the rubber liner.
Preparation of Artificial Vaginas for Semen Collection
Immediately before semen collection is attempted, the water jacket of the AV is usually filled with 45°C to 50°C water to provide an internal AV temperature of 44°C to 48°C (Figure 12-6). Providing an AV temperature above that of the body seems to aid in penile stimulation and facilitates ejaculation. Occasionally, some stallions may respond more favorably to semen collection with an AV if its luminal temperature is 50°C to 55°C. Sperm can be permanently damaged, however, by contact with surfaces above 45°C. As mentioned previously, the luminal temperature of a Missouri model AV can exceed 45°C without damaging sperm, provided that the glans penis protrudes beyond the water jacket when ejaculation occurs.
Pressure of the AV should be adjusted to provide uniformly good contact around the penis, without interfering with penile penetration. Proper AV pressure accommodates expansion of the penis to full erection. Full insertion of the penis into the AV during the first penile thrust and then maintenance of the penis in this fully inserted position is important; otherwise, the glans penis dilates and may be too large to permit full penile penetration into the AV. The result is extended contact of ejaculated semen with the AV liner and elevated temperature en route to the semen receptacle or ejaculatory failure from inadequate penile stimulation. Both temperature and water pressure in the AV should be maintained relatively constant during semen collection to promote consistent stallion performance and maximal sperm harvest.
The inner surface of the AV should be lubricated with a sterile, nonspermicidal lubricant before penile insertion. The collection receptacle (Figure 12-7) should be maintained at body temperature during semen collection and transport to the laboratory to prevent cold shock to the sperm before they are placed in a protective extender. Semen should also be protected from light.
To maximize the number of sperm available from each semen collection, an appropriate filter should be incorporated into the collection receptacle. The filter allows most of the gel-free fractions to pass into the seminal receptacle but traps the gel (which is presented in the final fractions of an ejaculate). Although some sperm inevitably are trapped in the gel and filter, more would be lost if the semen were filtered after collection of the combined gel and gel-free portions or if the gel were aspirated from the gel-free portion with a syringe. Nylon micromesh filters (see Figure 12-7) are superior to polyester matte filters for separating gel from gel-free fractions because they are nonabsorptive and do not trap as many sperm. The filter with its contained gel should be removed immediately on collection of the semen to prevent seepage of gel into the gel-free portion of the ejaculate.
Use of Condoms for Semen Collection
A condom (Figure 12-8) is a poor alternative to an AV for semen collection but may be the only viable option if the stallion will not breed an AV or if an AV is unavailable. Stallions most reluctant to breed an AV are those accustomed to breeding mares by natural service and occasionally, those that have never bred before. The quality of semen collected in a condom is inferior to that obtained with an AV because of the marked contamination of the sample with bacteria and debris from the exterior of the penis.
Preparation of Stallion Mount
Semen collection with an AV ordinarily is performed by allowing the stallion to mount a mare or breeding phantom. In certain situations, however, stallions have been trained to ejaculate with an AV or manual stimulation while standing on the ground (Figure 12-9). To train a stallion for ground collection, the stallion is teased to erection, and the penis is washed and dried. The stallion is approached by the collector from the left shoulder, and the AV is placed on the stallion’s penis. The AV is pushed toward the base of the penis to encourage thrusting. Stallions usually ejaculate after 5 to 10 pelvic thrusts. If the stallion does not ejaculate after the first attempt, the procedure is repeated until successful. As an alternative to ground collection, some practitioners prefer to use thin plastic mitts or rectal sleeves instead of an AV. The mitt is loosely placed on the penile shaft with 6 to 8 inches hanging beyond the end of the glans. Excess air is expelled from the mitt, and gentle pressure is applied to the glans penis to stimulate thrusting until ejaculation occurs. Application of hot compresses (6-inch squares of folded towels dipped in 50°C to 55°C water, squeezed to remove excess water from the compress), with one hand on the glans penis and one hand on the base of the penis, is often helpful in stimulating pelvic thrusting and ejaculation. A consistent, uniform method to which the individual stallion responds should be adopted. A modification of this technique can be used for chemical ejaculation (Figure 12-10). Chemical ejaculation is the method we use most often for stallions with ejaculatory problems associated with neurologic or musculoskeletal disorders that preclude mounting.
 Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|