Defining pain in the veterinary patient
The concept of pain is very important to the client, and in many instances one of the first questions on which they will want reassurance is: ‘Is (s)he in pain, because I do not want him/her to suffer?’. The ‘him or her’ in question is usually a very bouncy Golden Retriever that you are having difficulty in examining because (s)he is so lively! However, this does not mean that your very lively patient may not be experiencing pain.
How do we define pain in the veterinary cancer patient and how important is it?
One definition of pain is the unpleasant sensory and emotional experience associated with actual or potential tissue damage (Merskey & Bogduck 1994). There is considerable potential for tissue disruption, pain and suffering with the effects of cancer and with cancer treatment. For example, significant surgical pain may result from wide resections, the creation of large deficits and use of reconstructive techniques. Chronic, insidious, debilitating, continuous dull pain may also result from the effect of cancer on the body. Effective pain management is a vital part of the prolongation of good quality of life, and part of the ethical treatment of our patients (Lascelles & Main 2002).
To understand how to manage pain in our patients it is necessary to understand the different types of pain, the neural pathways involved, what activates these pathways and the clinical signs that indicate pain.
Assessment of pain
Assessment of pain in veterinary patients can be difficult. Animals may not show you they are in pain, and behavioural patterns are a guide only. For example, just because a dog eats, wants a pat and wags its tail does not mean it is not in pain. It is important to recognize that animals instinctively hide pain around people and other animals.
In many cases the presence of pain is subjective and based on behavioural changes that may be subtle and not apparent in the consulting room, but may have caused your client some concern – for example, vocalization, biting/aggression when an area is touched, restlessness, decreased activity, trembling, social withdrawal, changes in eating/drinking/sleeping/elimination patterns, changes in facial expression, biting/licking/guarding or not using a body part.
Objective assessments of pain such as heart and respiration rate, temperature, mucous membrane colour, blood cortisol and adrenaline levels, blood pressure and other physiological responses can also be a guide to pain levels in animals. However, these can be influenced by other factors such as stress, excitement and anxiety and are not always accurate (Conzemius et al, 1994 and Conzemius et al, 1997).
Firstly, it is important to address the degree and type of pain that an individual is experiencing.
Degree of pain
• Mild: may not significantly change patterns of behaviour and may go unnoticed both by the client and the veterinary professional.
• Moderate: will often result in some degree of altered behaviour and the patient will benefit from analgesics.
• Severe: requires immediate intervention with analgesics.
Surgical pain
Surgical pain warrants a special mention. All surgery causes pain unless it is treated! It is the responsibility of the surgeon to ensure that all patients are provided with adequate analgesia. This needs to be planned, pre-empted and well thought out. An effective surgical analgesic protocol begins with the premedication and ends when the animal no longer needs it.
The acute pain associated with cancer surgery should not be underestimated. Aggressive resections such as limb amputation and limb-sparing techniques, maxillectomy/mandibulectomy, chest wall resection, nasal planectomy, skin reconstruction techniques, etc. cause considerable tissue damage. Postoperative pain may need to be addressed for up to several weeks.
Acute or chronic pain
Chronic pain is debilitating but because the patient comes to terms with it, it may not be obvious to the veterinary professional, whereas the pain associated with trauma is acute, severe and apparent.
The effects of cancer on the body may also cause chronic pain, which may be insidious/subtle in onset and may be overlooked by the client or veterinarian. Consider the complex pain of a patient with appendicular osteosarcoma. We know that the pain associated with bone tumours is part somatic and part neuropathic, resulting in chronic pain that may only be visualized in the patient by intermittent lameness (moderate); however, if the dog falls and fractures the leg he will experience acute, severe pain.
Three phases of pain have been proposed by Cervero & Laird (1991).
• Phase 1: acute phase, with correspondingly short-lived response in the central nervous system.
• Phase 2: prolonged painful stimulation then leads to inflammation and continued discharge of peripheral nociceptors, with subsequent excitability of dorsal horn neurons. Sensitization may occur at either the peripheral (primary hyperalgesia) and/or the central level (central sensitization).
• Phase 3: peripheral nerve damage may lead to spontaneous discharge, which modifies (amplifies) the behaviour of dorsal horn neurons (secondary hyperalgesia), and allows peripheral nerves not normally associated with pain to access the ascending pain system and thereby evoke pain.
When a normally non-painful stimulus, even the slightest touch, causes severe pain due to central sensitization, the pain evoked is termed allodynia (‘wind-up pain’) (Brooks & Tracey 2005). Allodynia is usually due to previous inadequate or lack of pain treatment. The simultaneous use of multiple classes of analgesic drugs is often needed to reverse allodynia. These drugs are gradually withdrawn (the analgesic reverse pyramid approach) (Lascelles 2003).
Classification of pain (Lumb & Jones 1973)
Somatic pain
Somatic pain results in a dull or aching pain that is localized and comes from the stimulation of nociceptors present in cutaneous or deep tissues. Examples of somatic pain include metastatic bone pain and postsurgical incisional pain.
Visceral pain
Nociceptors are activated by infiltration, compression, extension or stretching of viscera. The pain is poorly localized and creates a deep, squeezing, pressure-like sensation. When acute it can be associated with autonomic dysfunction resulting in nausea, vomiting, etc. It is also often referred to cutaneous sites. The kappa-opioid receptors are involved in modulating visceral pain.
Neuropathic pain
Neuropathic pain is a consequence of injury to the central or peripheral nervous system and may result from tumour compression or infiltration of peripheral nerves or spinal cord. Injury to nerves caused by surgery, radiotherapy or chemotherapy can also result in neuropathic pain. Neuropathic pain can be severe.
Complex pain
Complex pain results from stimulation of more than one pathway – for example, bone pain is probably mixed pain involving both somatic and neuropathic pain sensations.
The neurotransmission of pain
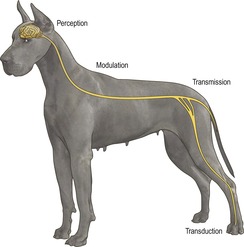
(See Figure 11.1 for an illustration of the neural pathways associated with pain.)
 |
| Figure 11.1 |
Analgesia is induced by the interruption of nociception (the pathway from pain stimulus to the central perception of pain). A multimodal approach using agents that work at different parts of the pain pathway gives added benefits (see Table 11.1).
| Nociception pathway | Drug class | Effect on pathway |
|---|---|---|
| Transduction | Opioids, NSAIDs, local anaesthetics | Inhibit peripheral sensitization of nociceptors |
| Transmission | Local anaesthetics, α2 agonists | At site/nerves targeting site or spinal canal May interrupt transmission All inhibit impulse conduction |
| Modulation | Opioids, local anaesthetics, α2 agonists, tricyclic antidepressants, cholinesterase inhibitors, NMDA antagonists, NSAIDs | Inhibit central sensitization of nociceptors |
| Perception | Opioids, benzodiazepines, α2 agonists | May block perception of pain |
| General anaesthesia | Blocks perception of pain |
Nociception pathway (Practical Pain Management 1988)
Transduction
This is the conversion of physical energy (noxious stimulus, e.g. mechanical, thermal, chemical) into electrical activity (a neuronal action potential) at the peripheral nociceptor (free nerve ending) (Wall 1989). Prostaglandins, bradykinin, leukotrienes and substance P released from damaged cells can directly stimulate nerve endings and can increase sensitivity to subsequent noxious stimuli and other components of the ‘inflammatory soup’ (Fields 1987).
Transmission
This is the movement of nerve impulses through afferent peripheral and central nervous systems.
• Peripheral nervous system: fast transmission through myelinated Aδ fibres (sharp mechanical stimuli) and slow through unmyelinated C fibres (dull, throbbing, longer-lasting pain) (Raja et al 1999).
• Central nervous system: pain impulse travels via peripheral nerves to dorsal horn of spinal cord (Basbaum & Jessell 2000). Pain processing (including hyperalgesia and allodynia) occurs in the dorsal horn. Pain-related information then ascends in the contralateral spinothalamic tract (and also direct connections to the medulla and brain stem via the spinoreticular and spinomesencephalic tracts, and to the hypothalamus via the spinohypothalamic tract) to the higher centres in the brain (Brooks & Tracey 2005).
Modulation
Modulation of the pain response occurs through endogenous analgesic systems that modify nociceptive transmission in the spinal column. The ‘gate theory’ is that the signal sent to the brain is the summation of excitatory and inhibitory impulses (Melzack & Wall 1965). Central sensitization of modulation can be due to the growth of nerve endings under the influence of chemical mediators that fire on their own without peripheral input (McMahon et al 1993). Thus the brain and spinal cord can not only modulate but also create pain perception (Brooks & Tracey 2005).
Perception
Perception is the integration of information in the cerebral cortex, allowing the conscious subjective and emotional experience of pain (Brooks & Tracey 2005).
A number of classes of drugs are available to the veterinary surgeon to interrupt the nociception pathway to provide analgesia (see Table 11.1 and ‘Classes of drugs’ below).
Rationale for the treatment of pain
Pain is deleterious to the wellbeing of any patient and veterinary professionals have a responsibility to provide appropriate analgesia for their patients.
Why is pain deleterious? (Hellyer & Fails 2003)
The deleterious effects of pain on the body are significant and include:
• increased neuroendocrine response, resulting in increased catecholamines, increased cortisol, increased glucagon and decreased anabolic hormones
• a negative nitrogen balance (catabolism)
• increased medullary stimulation, leading to increased sympathetic tone, increased cardiac workload (increased cardiac output and heart rate), increased respiratory rate and increased vascular resistance
• derangement of autonomic control of abdominal viscera, resulting in ileus and urinary dysfunction
• pulmonary dysfunction
• muscle fatigue.
What we see as a result of these physiological effects is distress and ultimately exhaustion, with the end result of prolonged convalescence and increased morbidity and mortality.
What are the aims of pain management? (Fleming 2001)
The ultimate goal of pain management is to make the patient as free of pain as possible. Practically, this means addressing the emotional and physiological aspects of pain by:
• decreasing nociception (block sensory input, modulate nociception signals)
• decreasing the central perception of pain
• decreasing fear and stress
• decreasing disability due to pain by mobilizing and motivating
• maintaining a comfortable environment and encouraging early interaction.
Guidelines for the rational use of analgesics in the management of cancer pain (Foley 2005)
1. Start with a specific drug for a specific type of pain.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


